Summary information and primary citation
- PDB-id
- 7pik; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- cryo-EM (2.68 Å)
- Summary
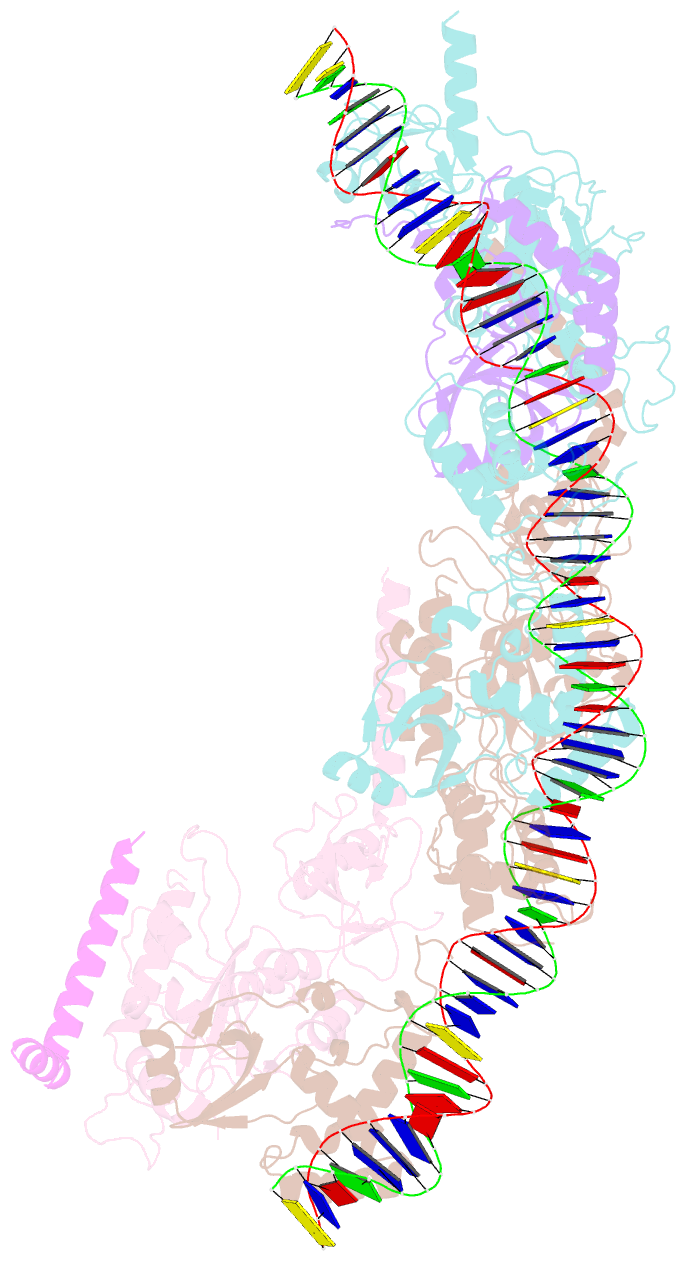
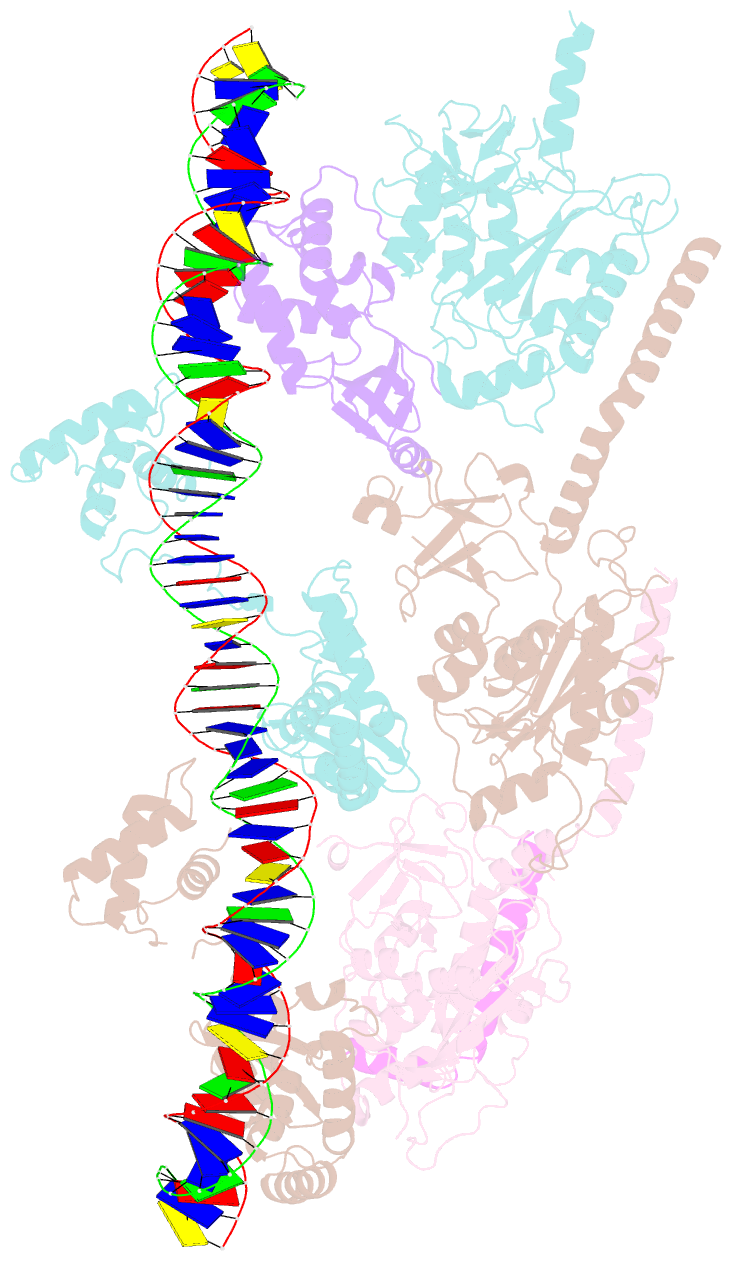
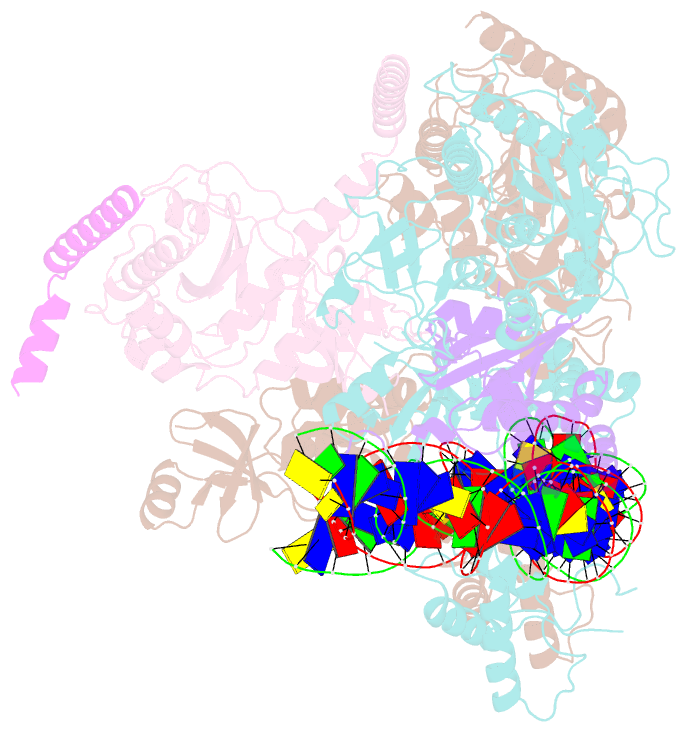
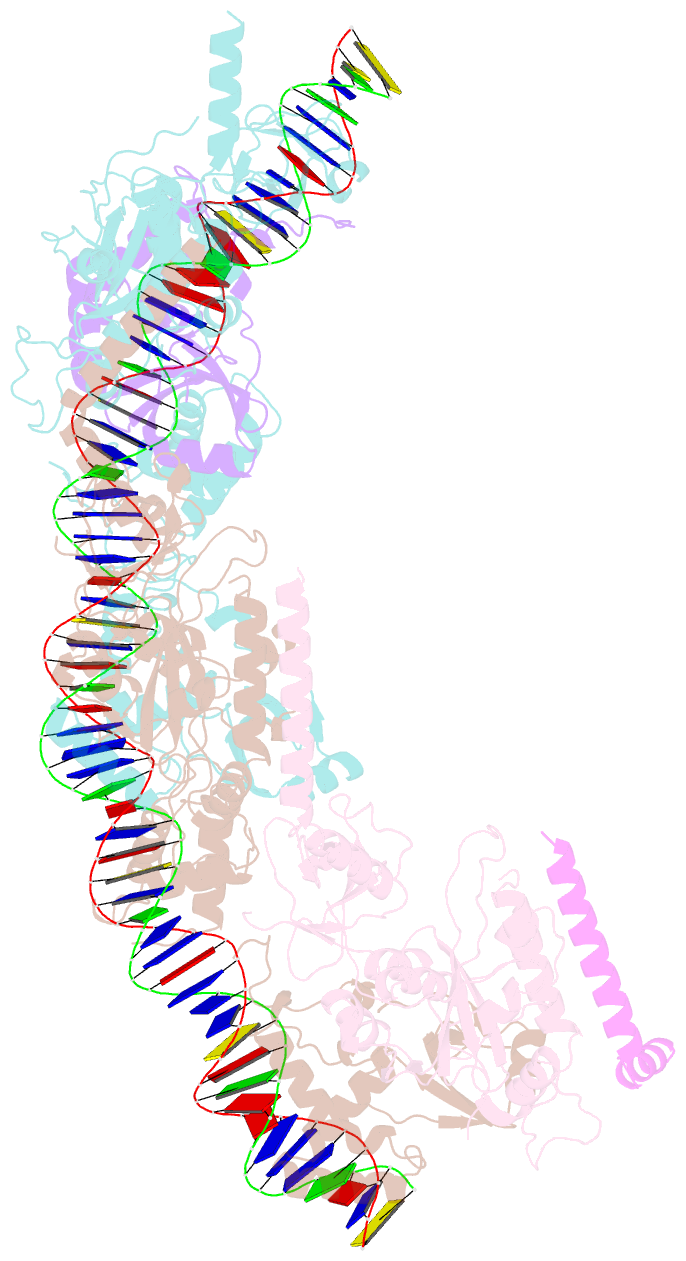
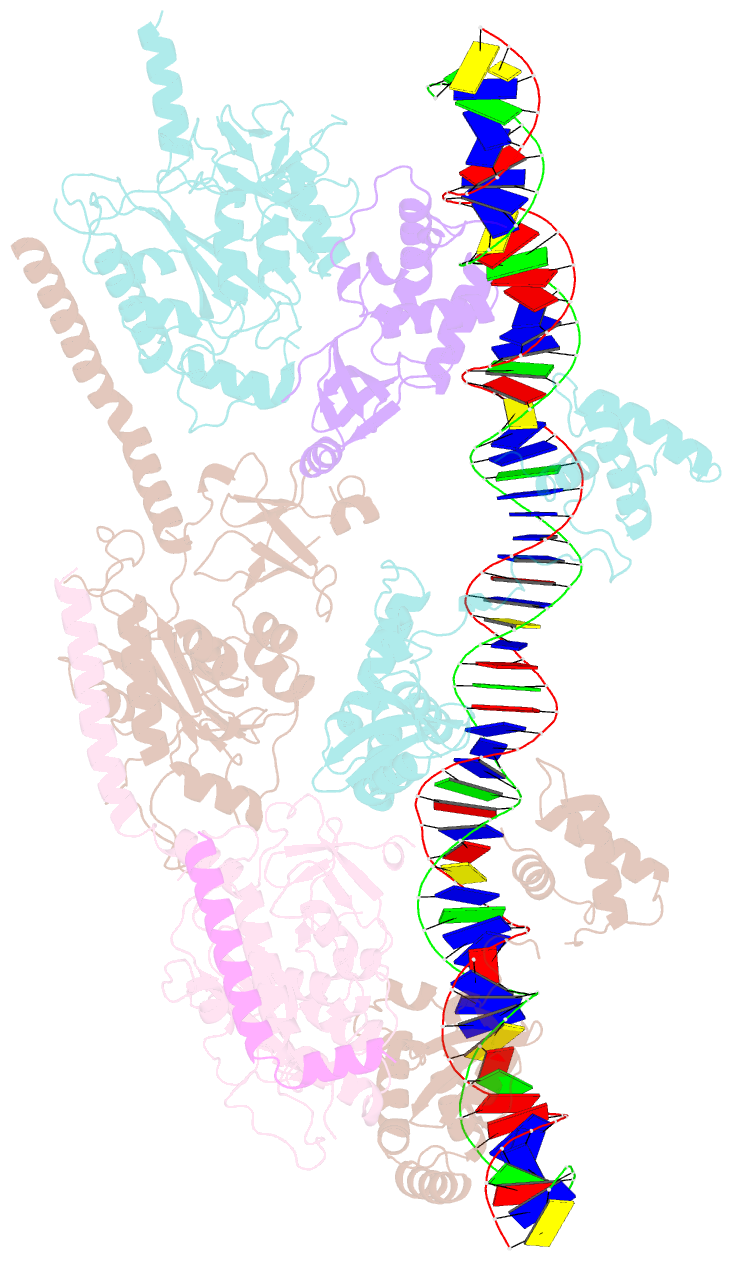
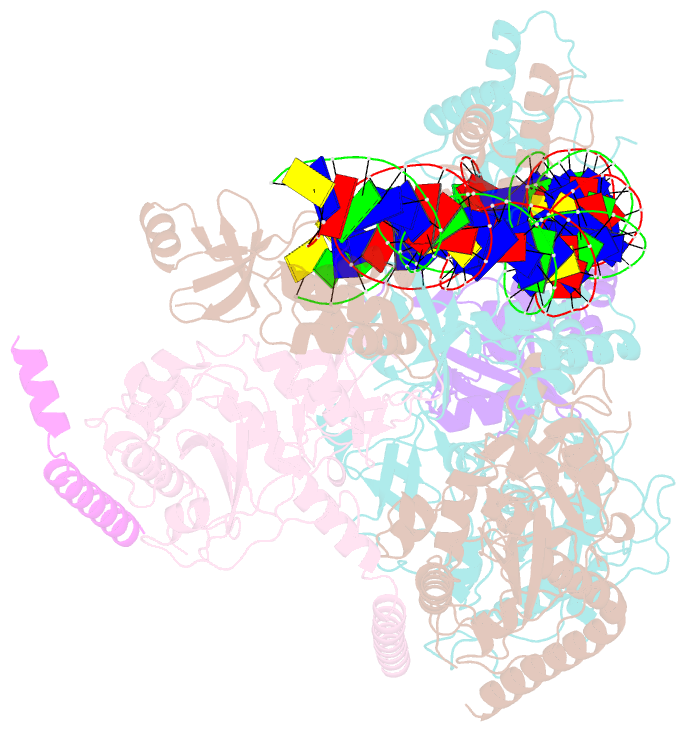
- cryo-EM structure of e. coli tnsb in complex with right end fragment of tn7 transposon
- Reference
- Kaczmarska Z, Czarnocki-Cieciura M, Gorecka-Minakowska KM, Wingo RJ, Jackiewicz J, Zajko W, Poznanski JT, Rawski M, Grant T, Peters JE, Nowotny M (2022): "Structural basis of transposon end recognition explains central features of Tn7 transposition systems." Mol.Cell, 82, 2618. doi: 10.1016/j.molcel.2022.05.005.
- Abstract
- Tn7 is a bacterial transposon with relatives containing element-encoded CRISPR-Cas systems mediating RNA-guided transposon insertion. Here, we present the 2.7 Å cryoelectron microscopy structure of prototypic Tn7 transposase TnsB interacting with the transposon end DNA. When TnsB interacts across repeating binding sites, it adopts a beads-on-a-string architecture, where the DNA-binding and catalytic domains are arranged in a tiled and intertwined fashion. The DNA-binding domains form few base-specific contacts leading to a binding preference that requires multiple weakly conserved sites at the appropriate spacing to achieve DNA sequence specificity. TnsB binding imparts differences in the global structure of the protein-bound DNA ends dictated by the spacing or overlap of binding sites explaining functional differences in the left and right ends of the element. We propose a model of the strand-transfer complex in which the terminal TnsB molecule is rearranged so that its catalytic domain is in a position conducive to transposition.