Summary information and primary citation
- PDB-id
- 7pu7; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- replication
- Method
- cryo-EM (2.9 Å)
- Summary
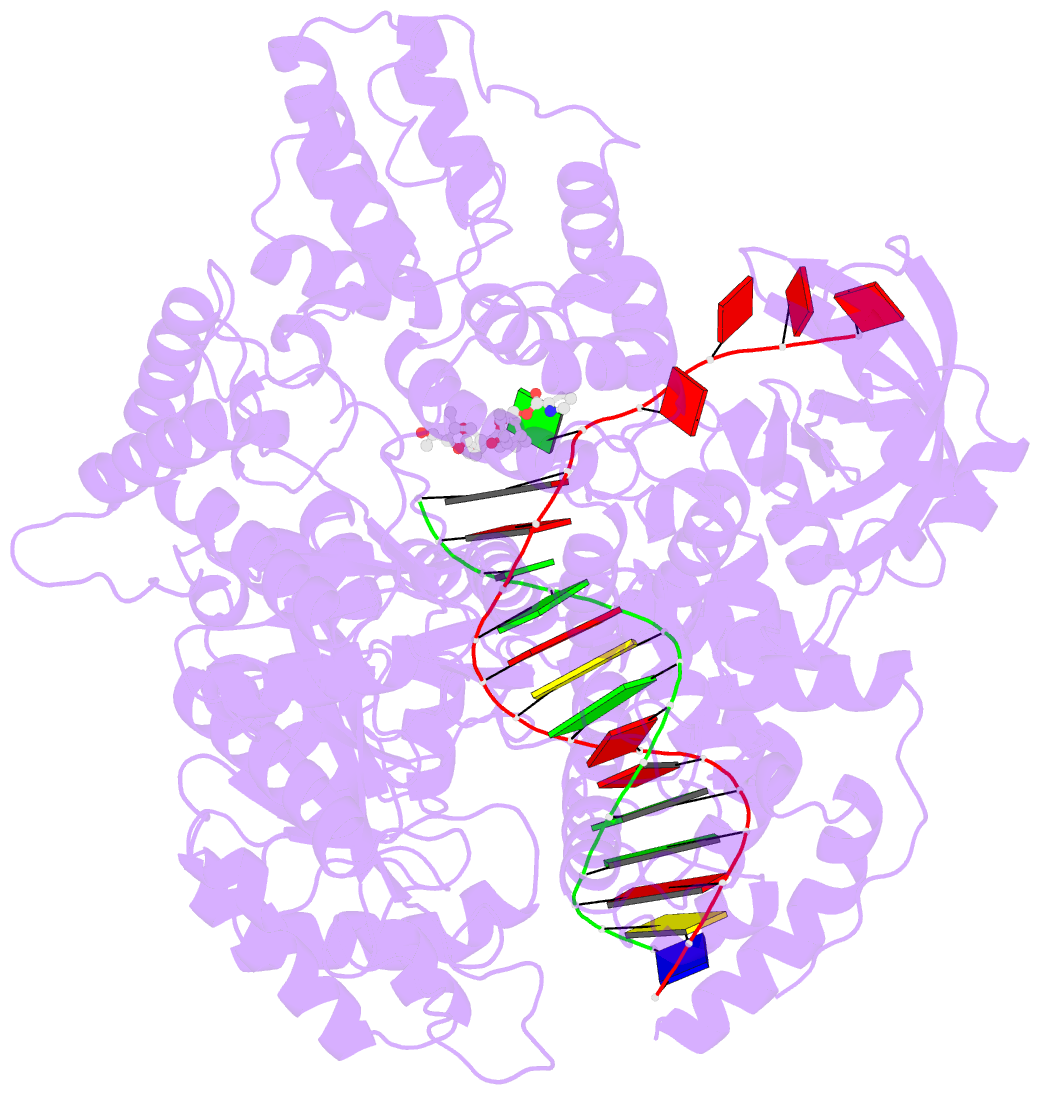
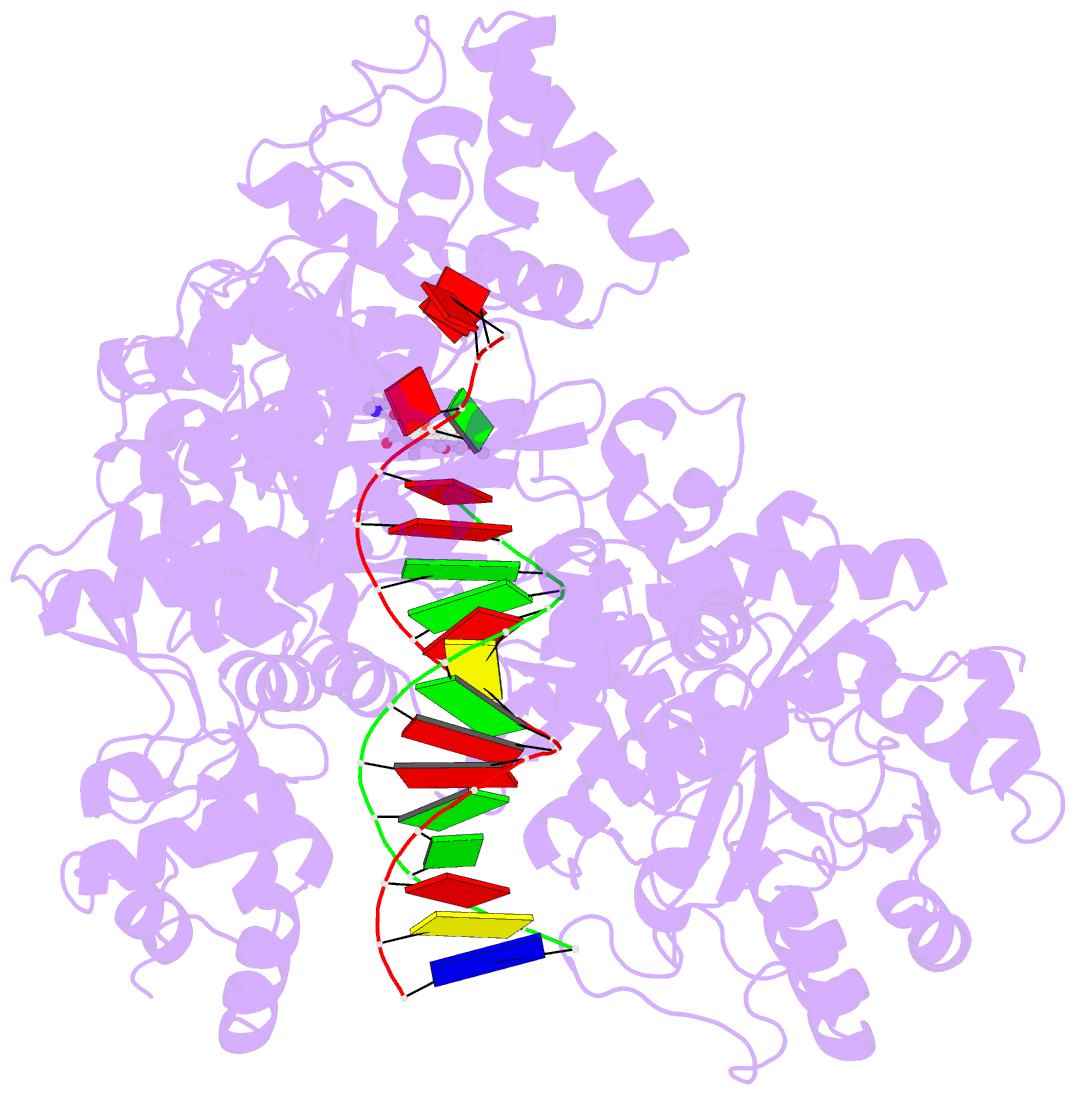
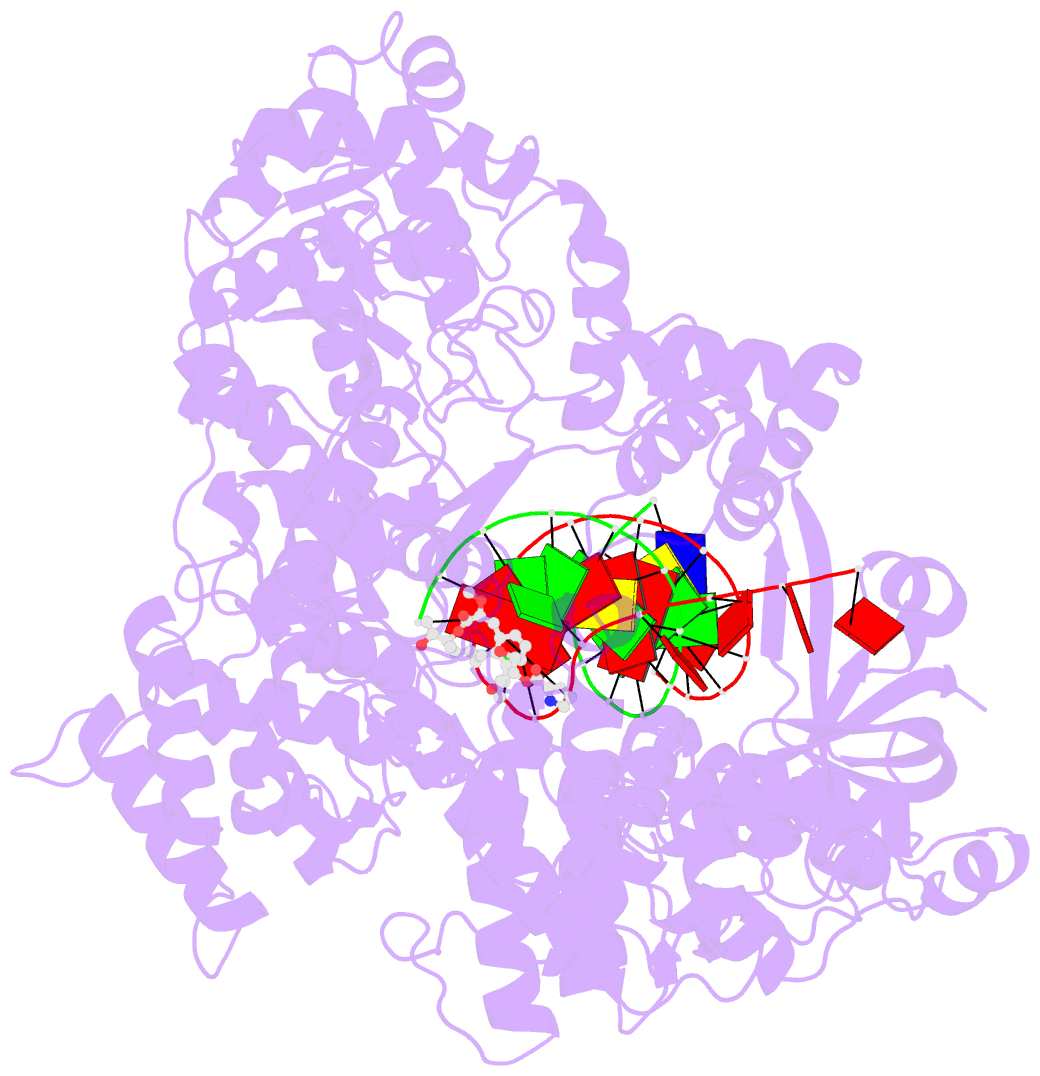
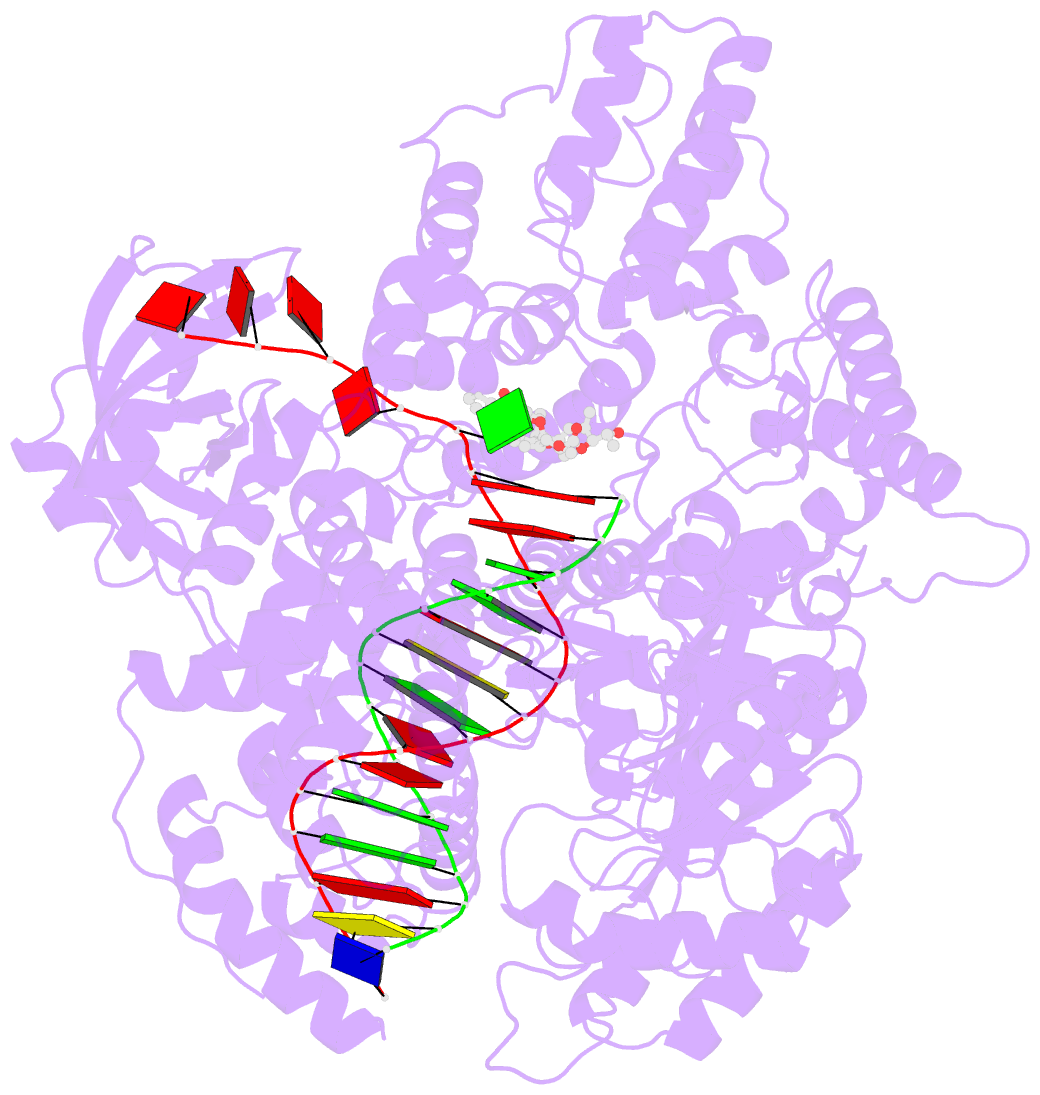
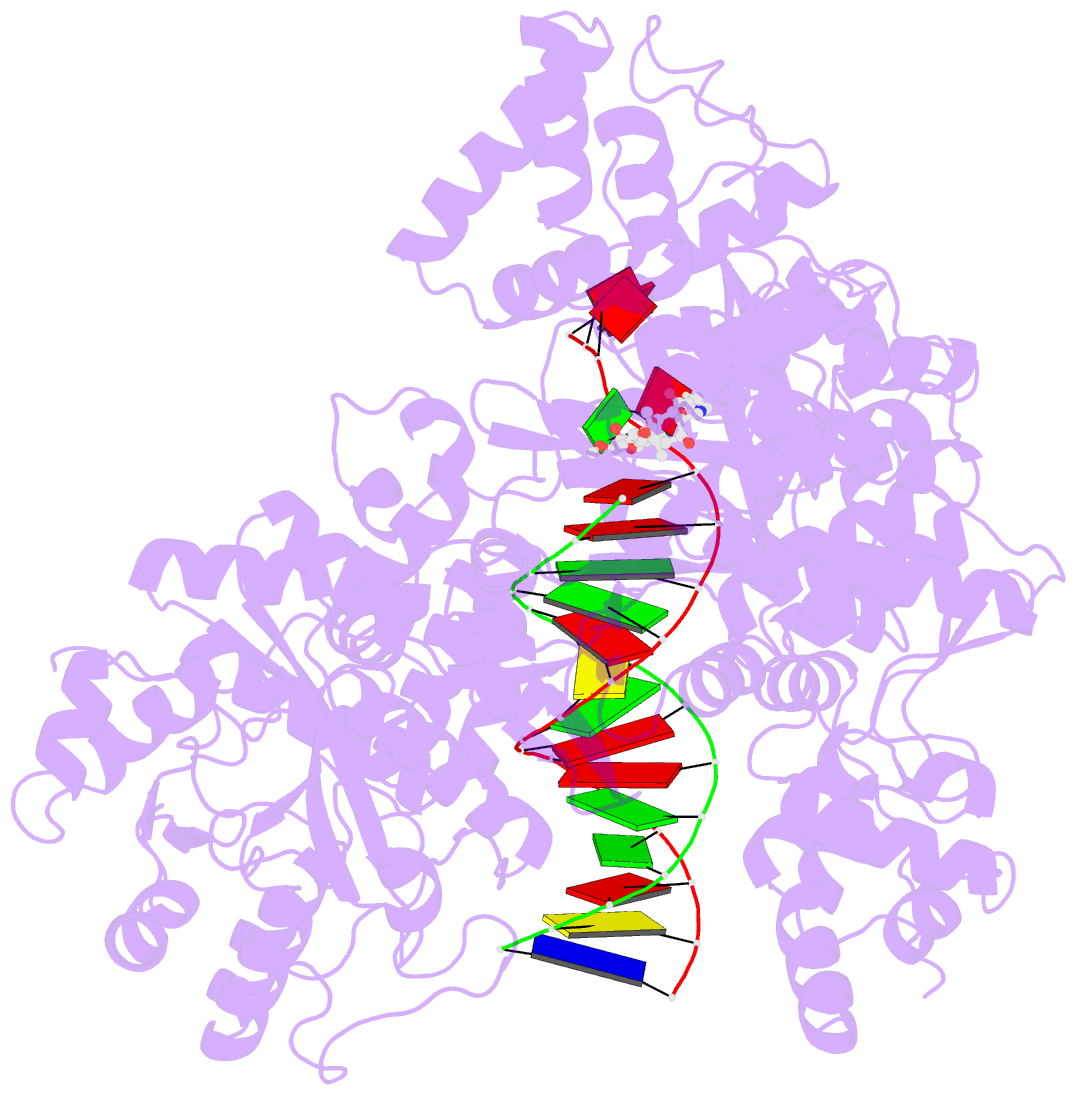
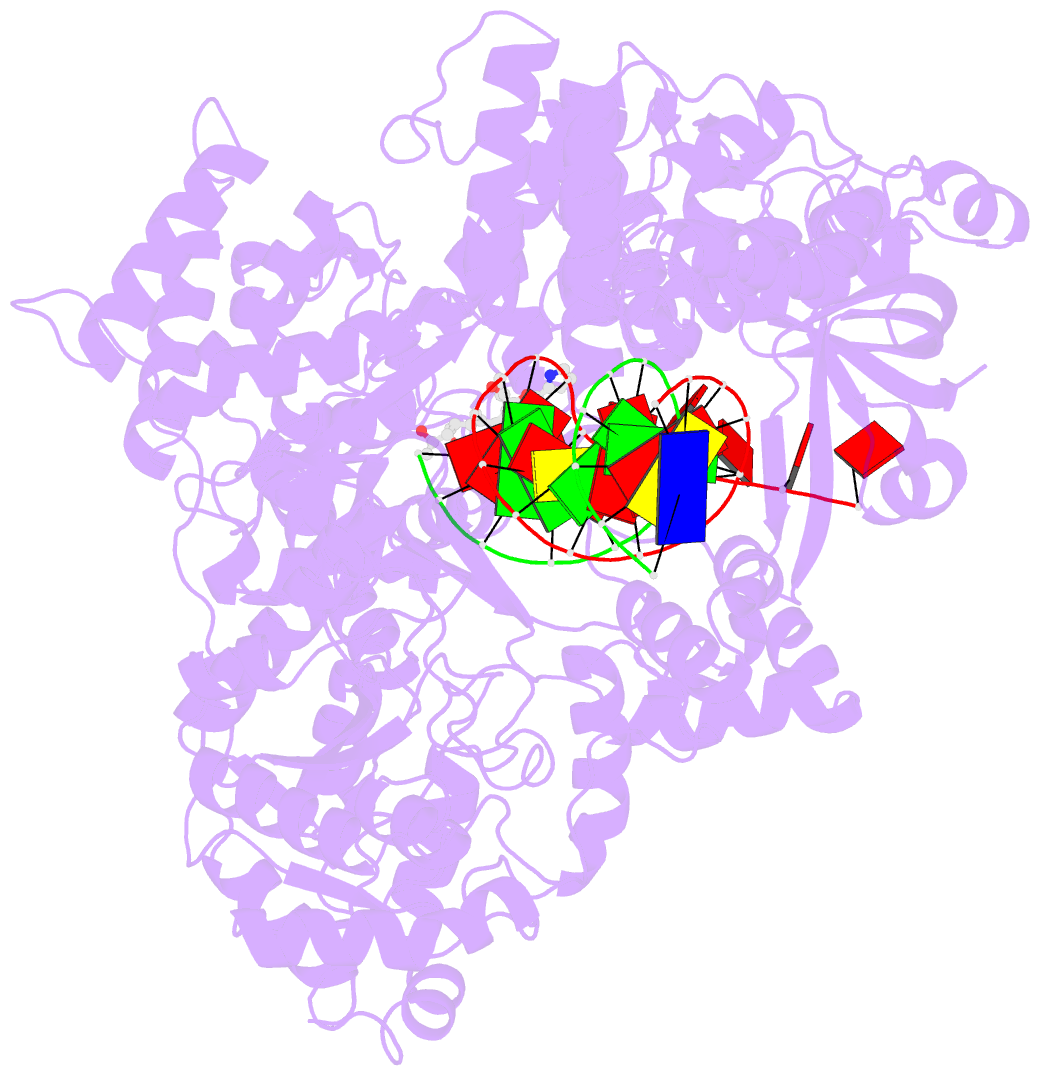
- DNA polymerase from m. tuberculosis
- Reference
- Chengalroyen MD, Mason MK, Borsellini A, Tassoni R, Abrahams GL, Lynch S, Ahn YM, Ambler J, Young K, Crowley BM, Olsen DB, Warner DF, Barry Iii CE, Boshoff HIM, Lamers MH, Mizrahi V (2022): "DNA-Dependent Binding of Nargenicin to DnaE1 Inhibits Replication in Mycobacterium tuberculosis." Acs Infect Dis., 8, 612-625. doi: 10.1021/acsinfecdis.1c00643.
- Abstract
- Natural products provide a rich source of potential antimicrobials for treating infectious diseases for which drug resistance has emerged. Foremost among these diseases is tuberculosis. Assessment of the antimycobacterial activity of nargenicin, a natural product that targets the replicative DNA polymerase of Staphylococcus aureus, revealed that it is a bactericidal genotoxin that induces a DNA damage response in Mycobacterium tuberculosis (Mtb) and inhibits growth by blocking the replicative DNA polymerase, DnaE1. Cryo-electron microscopy revealed that binding of nargenicin to Mtb DnaE1 requires the DNA substrate such that nargenicin is wedged between the terminal base pair and the polymerase and occupies the position of both the incoming nucleotide and templating base. Comparative analysis across three bacterial species suggests that the activity of nargenicin is partly attributable to the DNA binding affinity of the replicative polymerase. This work has laid the foundation for target-led drug discovery efforts focused on Mtb DnaE1.