Summary information and primary citation
- PDB-id
- 7qh6; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- cryo-EM (3.08 Å)
- Summary
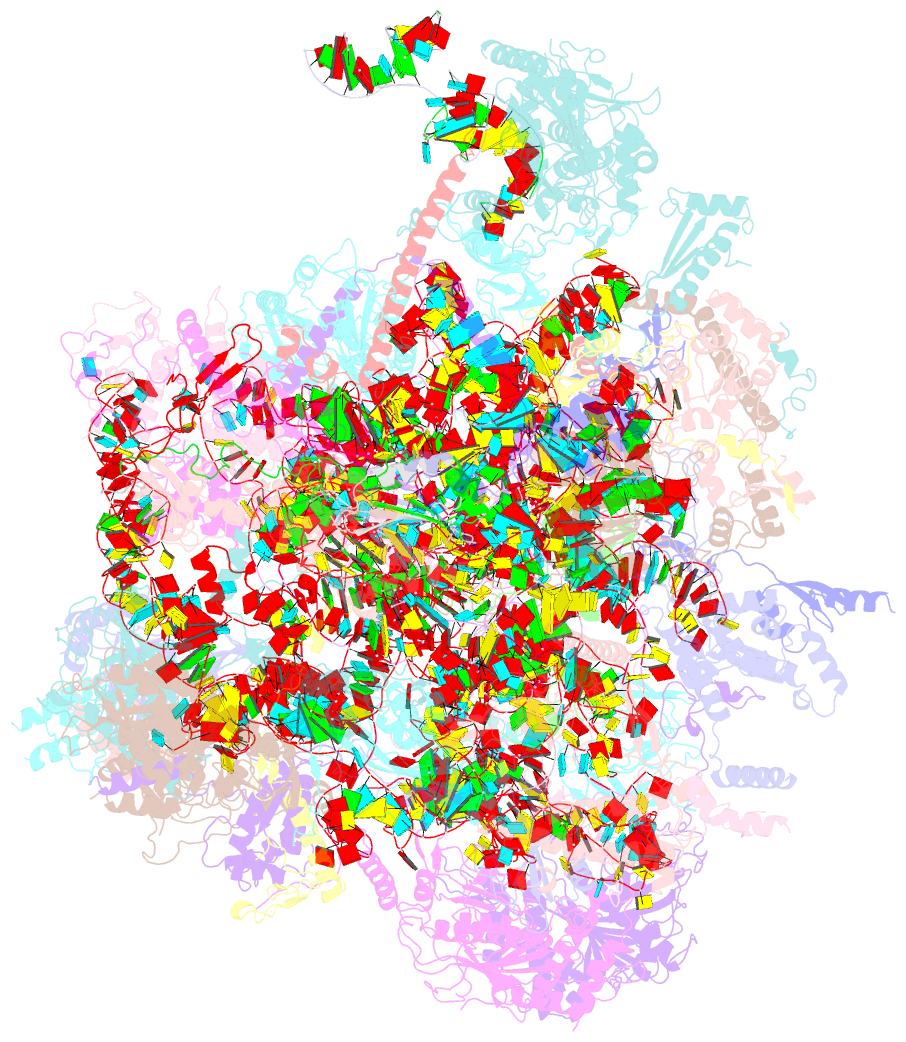
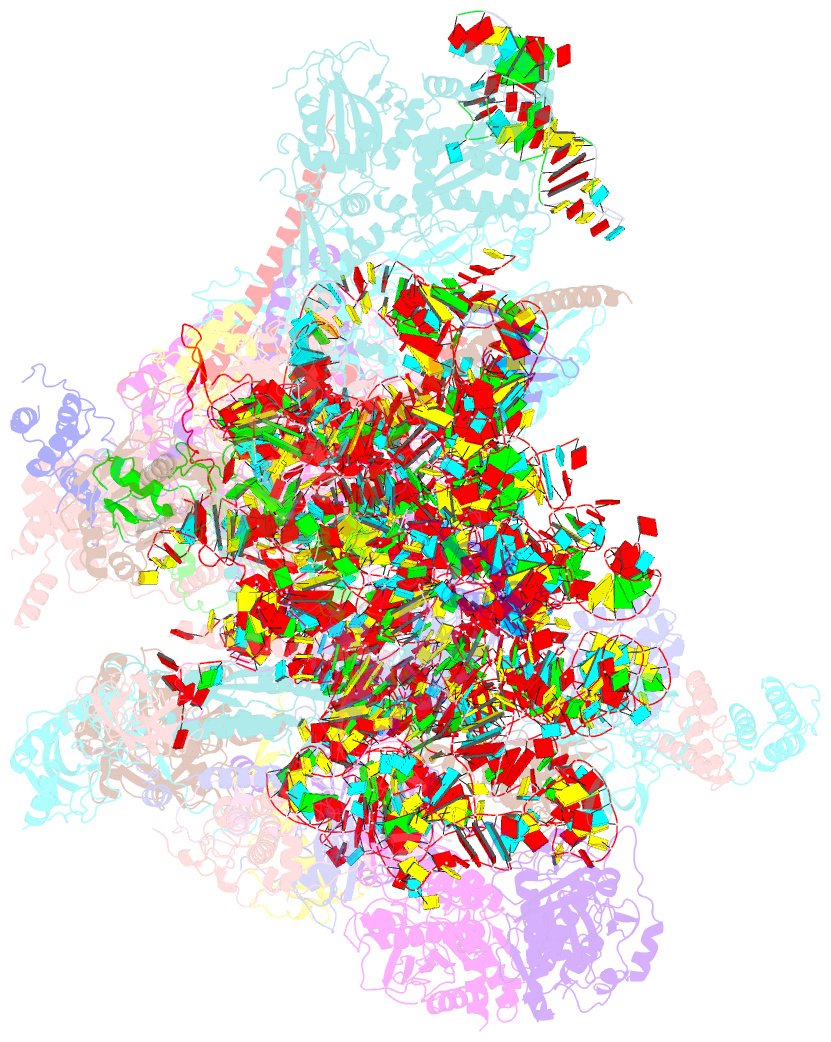
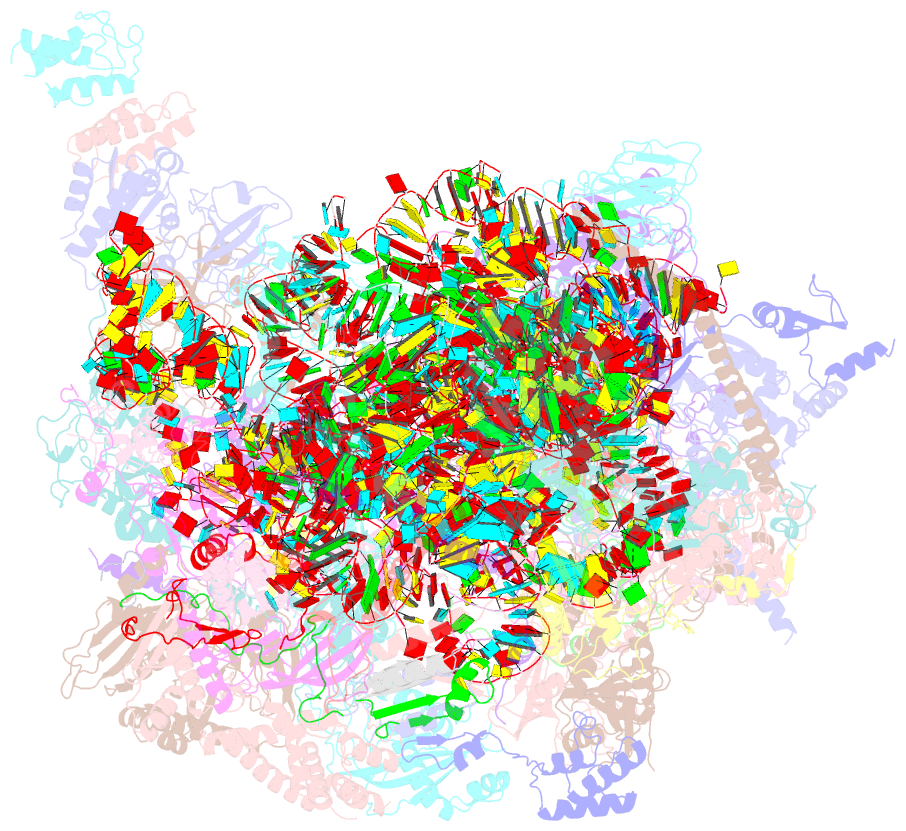
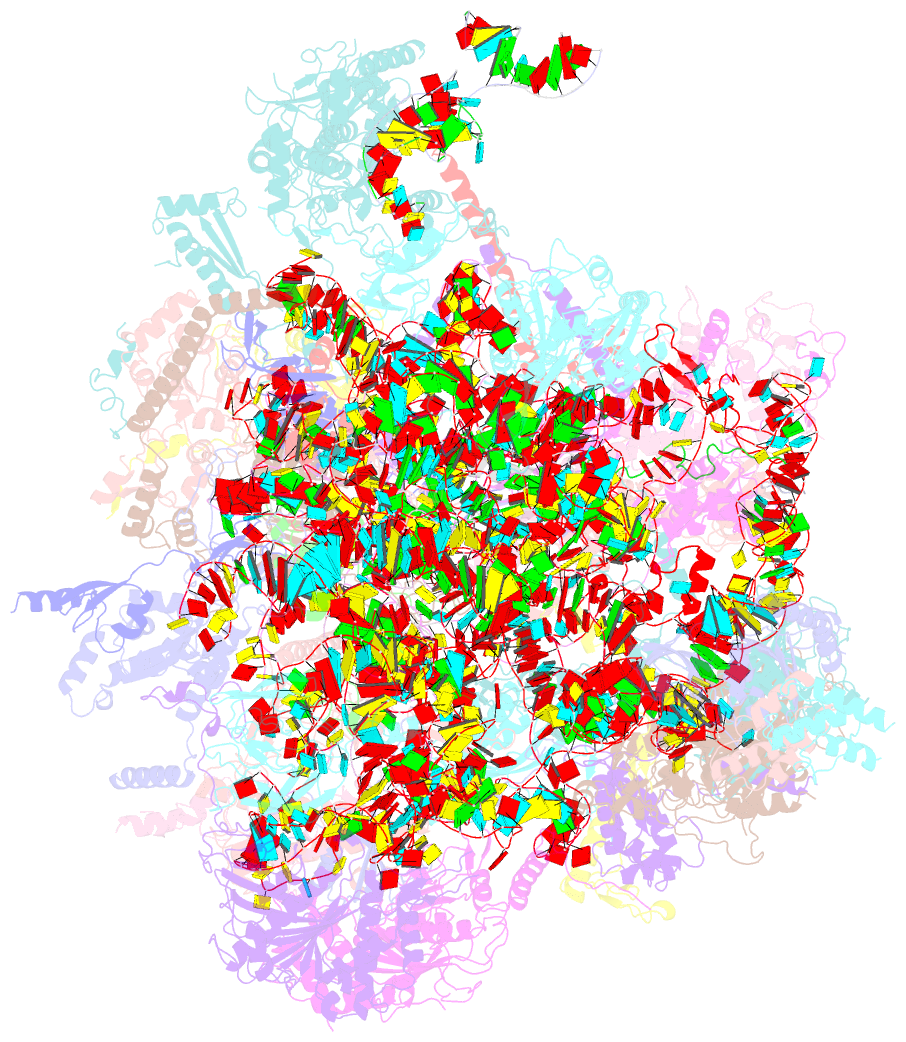
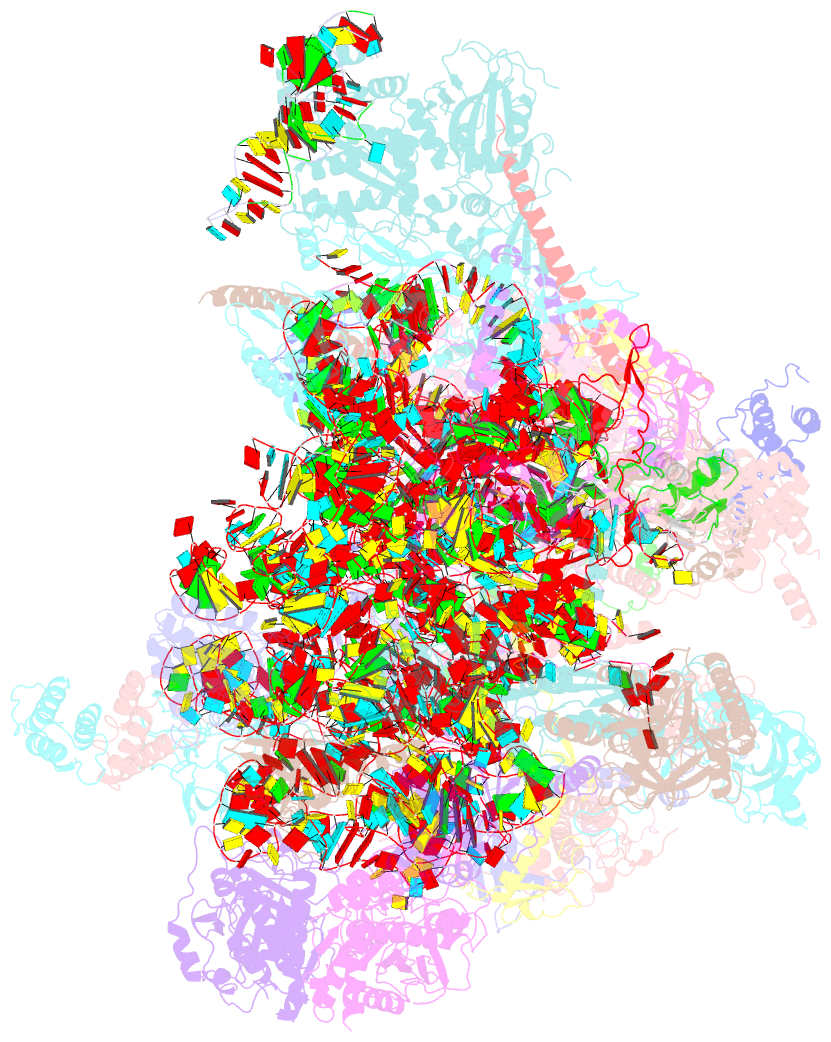
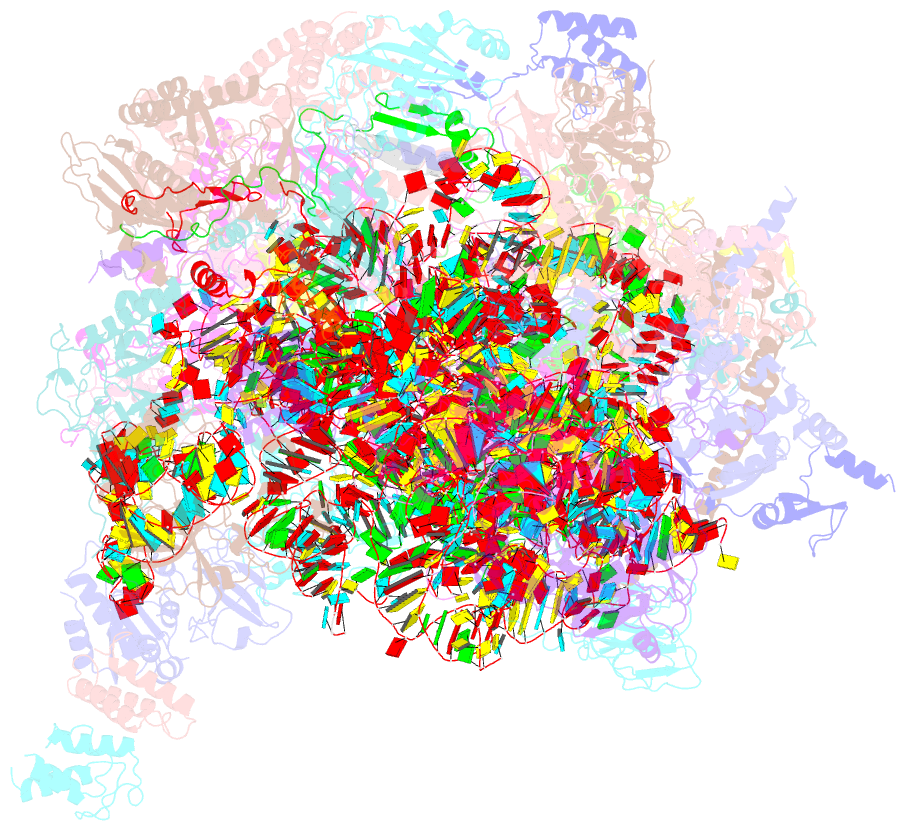
- cryo-EM structure of the human mtlsu assembly intermediate upon mrm2 depletion - class 1
- Reference
- Rebelo-Guiomar P, Pellegrino S, Dent KC, Sas-Chen A, Miller-Fleming L, Garone C, Van Haute L, Rogan JF, Dinan A, Firth AE, Andrews B, Whitworth AJ, Schwartz S, Warren AJ, Minczuk M (2022): "A late-stage assembly checkpoint of the human mitochondrial ribosome large subunit." Nat Commun, 13, 929. doi: 10.1038/s41467-022-28503-5.
- Abstract
- Many cellular processes, including ribosome biogenesis, are regulated through post-transcriptional RNA modifications. Here, a genome-wide analysis of the human mitochondrial transcriptome shows that 2'-O-methylation is limited to residues of the mitoribosomal large subunit (mtLSU) 16S mt-rRNA, introduced by MRM1, MRM2 and MRM3, with the modifications installed by the latter two proteins being interdependent. MRM2 controls mitochondrial respiration by regulating mitoribosome biogenesis. In its absence, mtLSU particles (visualized by cryo-EM at the resolution of 2.6 Å) present disordered RNA domains, partial occupancy of bL36m and bound MALSU1:L0R8F8:mtACP anti-association module, allowing five mtLSU biogenesis intermediates with different intersubunit interface configurations to be placed along the assembly pathway. However, mitoribosome biogenesis does not depend on the methyltransferase activity of MRM2. Disruption of the MRM2 Drosophila melanogaster orthologue leads to mitochondria-related developmental arrest. This work identifies a key checkpoint during mtLSU assembly, essential to maintain mitochondrial homeostasis.