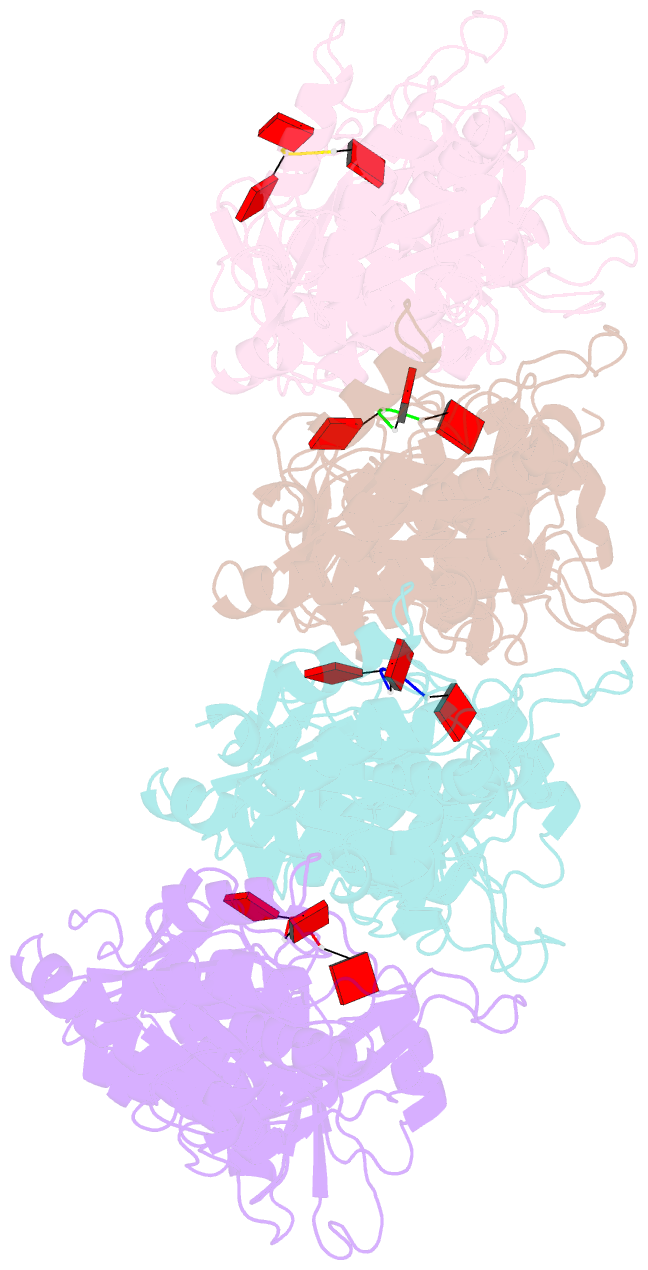
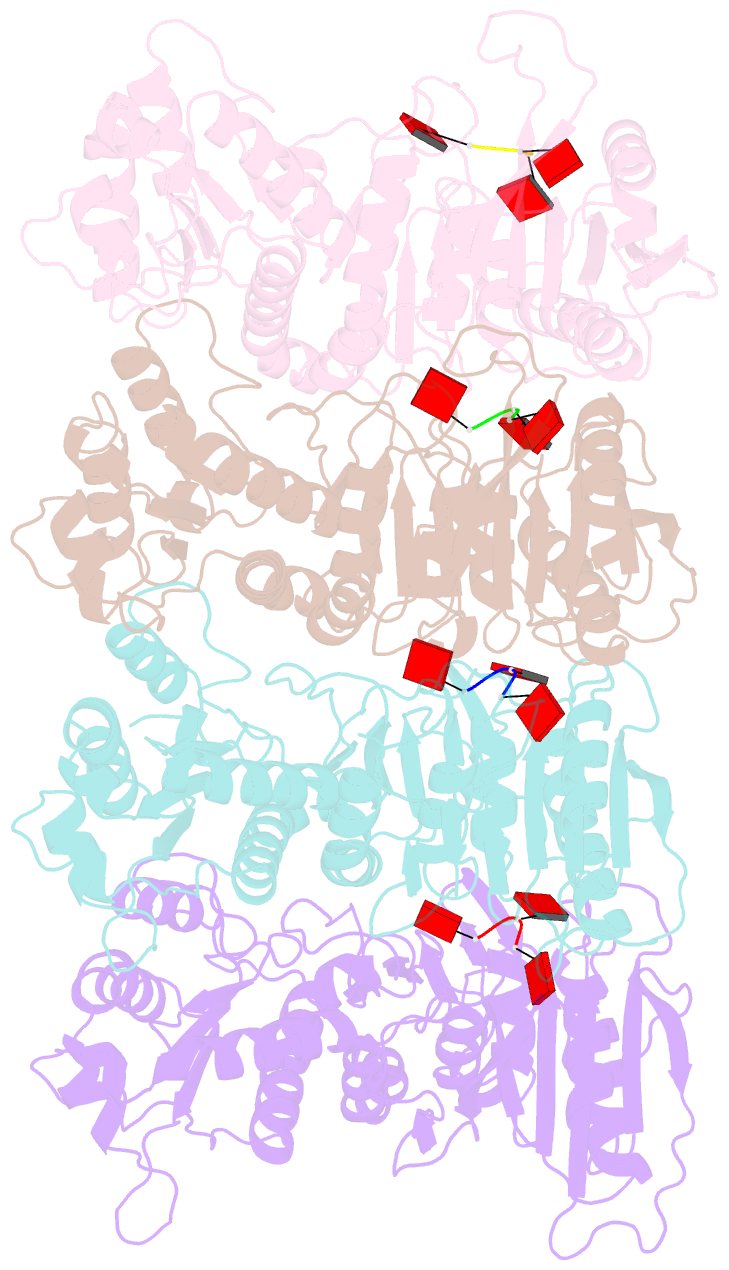
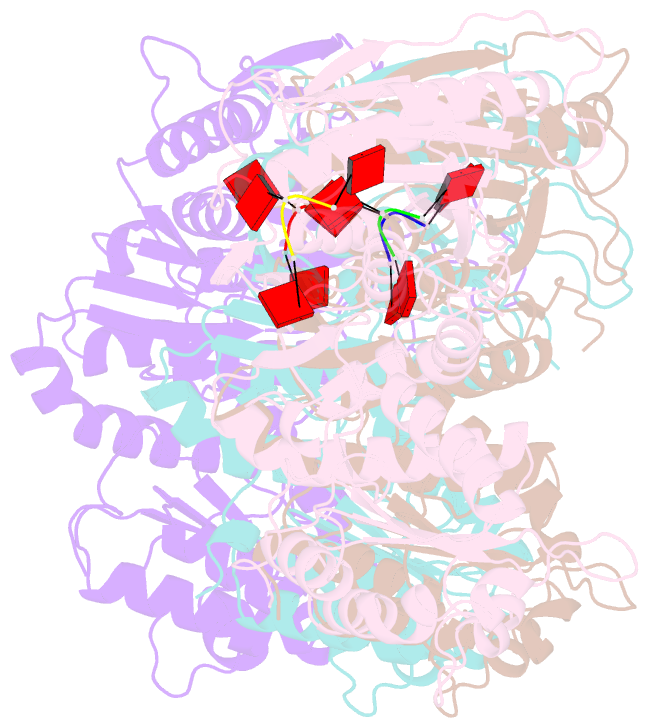
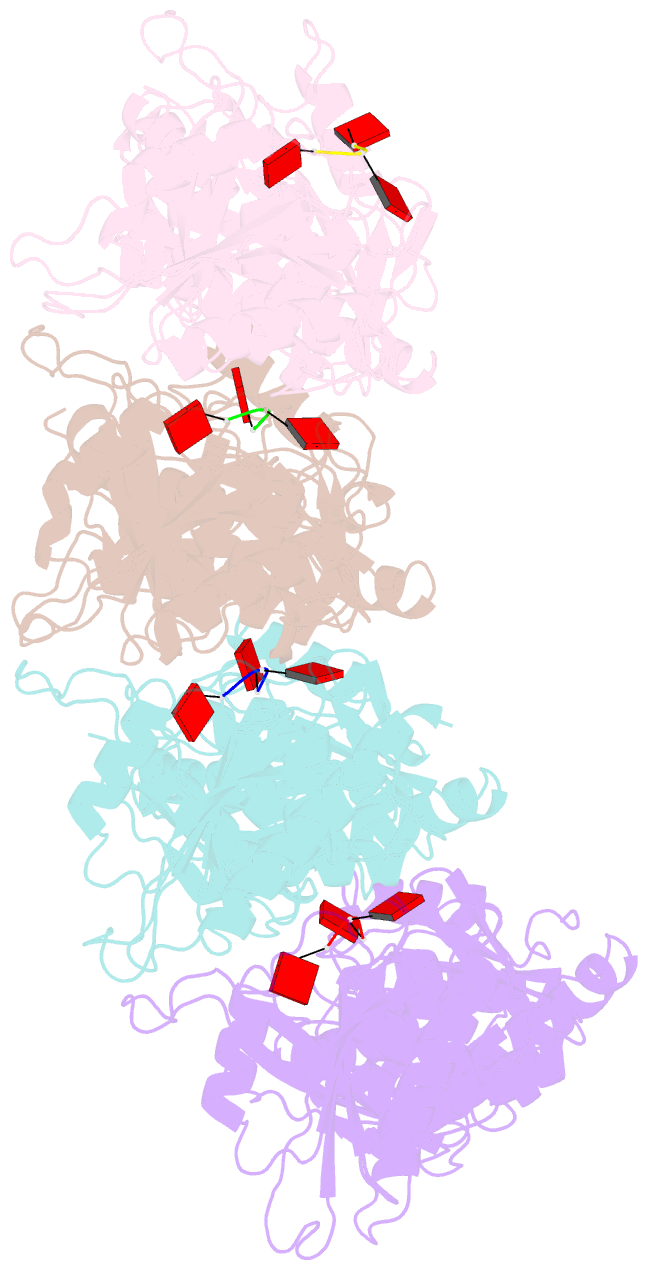
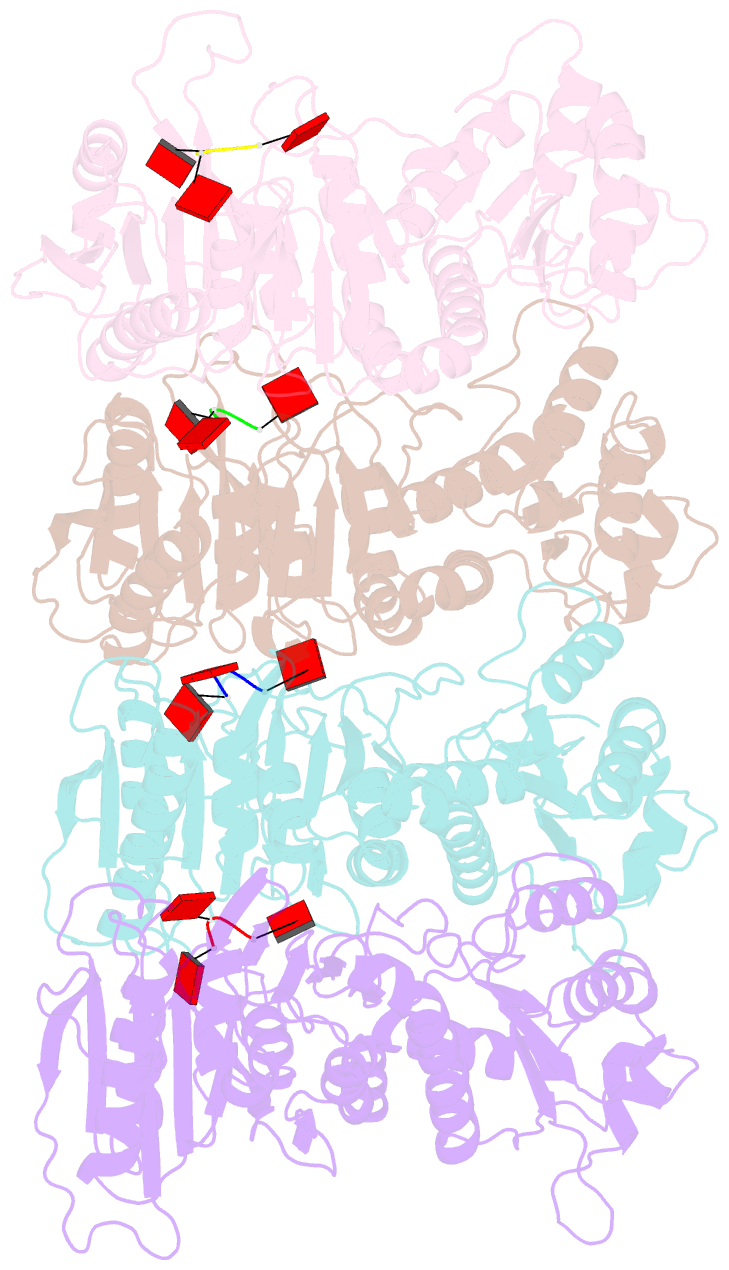
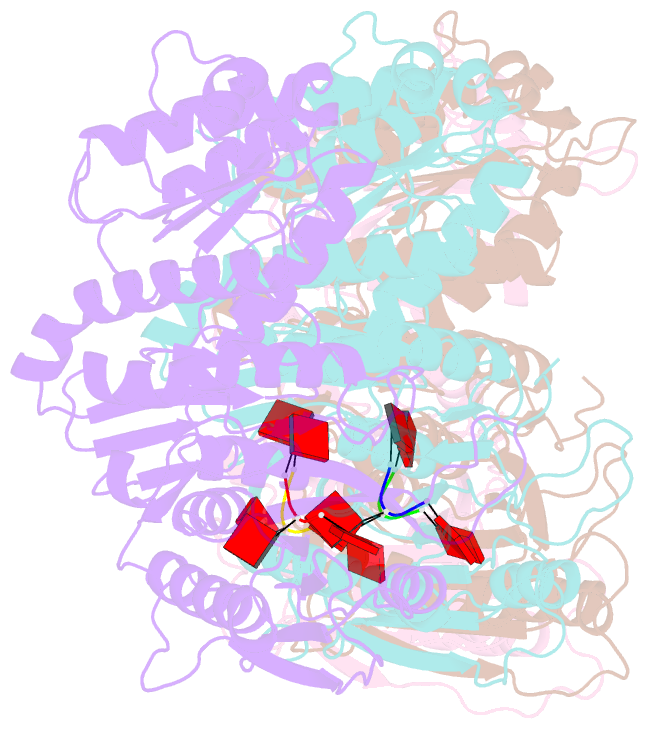
Summary information and primary citation
- PDB-id
- 7qqk; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- signaling protein
- Method
- cryo-EM (3.8 Å)
- Summary
- Tir-saved effector bound to ca3
- Reference
- Hogrel G, Guild A, Graham S, Rickman H, Gruschow S, Bertrand Q, Spagnolo L, White MF (2022): "Cyclic nucleotide-induced helical structure activates a TIR immune effector." Nature, 608, 808-812. doi: 10.1038/s41586-022-05070-9.
- Abstract
- Cyclic nucleotide signalling is a key component of antiviral defence in all domains of life. Viral detection activates a nucleotide cyclase to generate a second messenger, resulting in activation of effector proteins. This is exemplified by the metazoan cGAS-STING innate immunity pathway1, which originated in bacteria2. These defence systems require a sensor domain to bind the cyclic nucleotide and are often coupled with an effector domain that, when activated, causes cell death by destroying essential biomolecules3. One example is the Toll/interleukin-1 receptor (TIR) domain, which degrades the essential cofactor NAD+ when activated in response to infection in plants and bacteria2,4,5 or during programmed nerve cell death6. Here we show that a bacterial antiviral defence system generates a cyclic tri-adenylate that binds to a TIR-SAVED effector, acting as the 'glue' to allow assembly of an extended superhelical solenoid structure. Adjacent TIR subunits interact to organize and complete a composite active site, allowing NAD+ degradation. Activation requires extended filament formation, both in vitro and in vivo. Our study highlights an example of large-scale molecular assembly controlled by cyclic nucleotides and reveals key details of the mechanism of TIR enzyme activation.