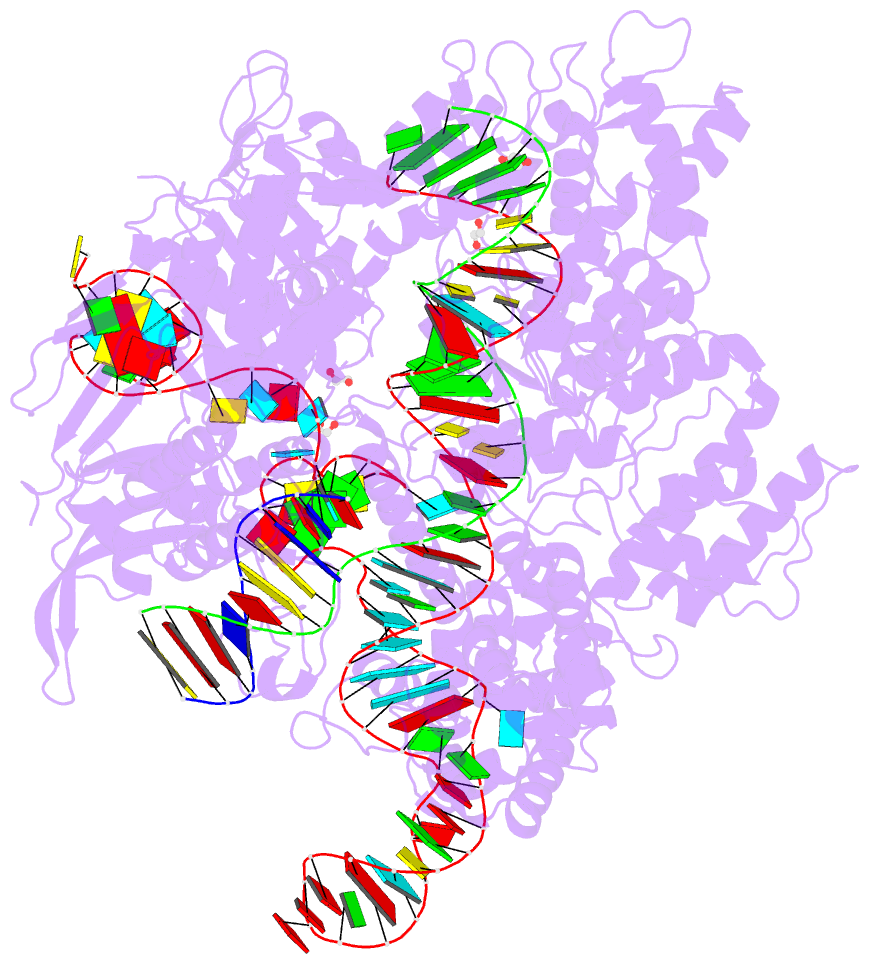
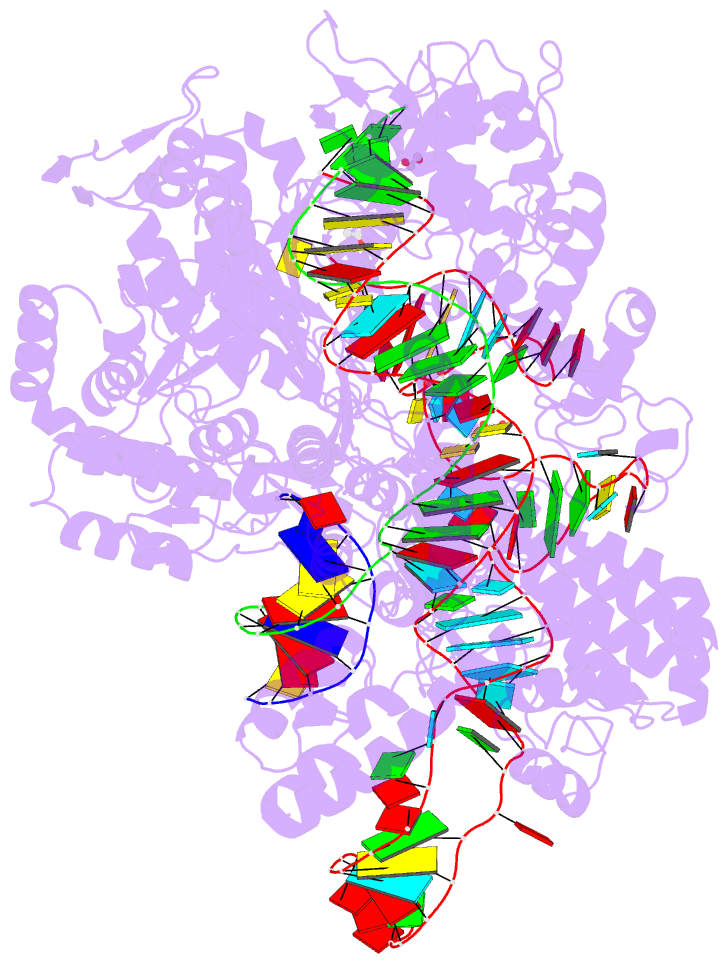
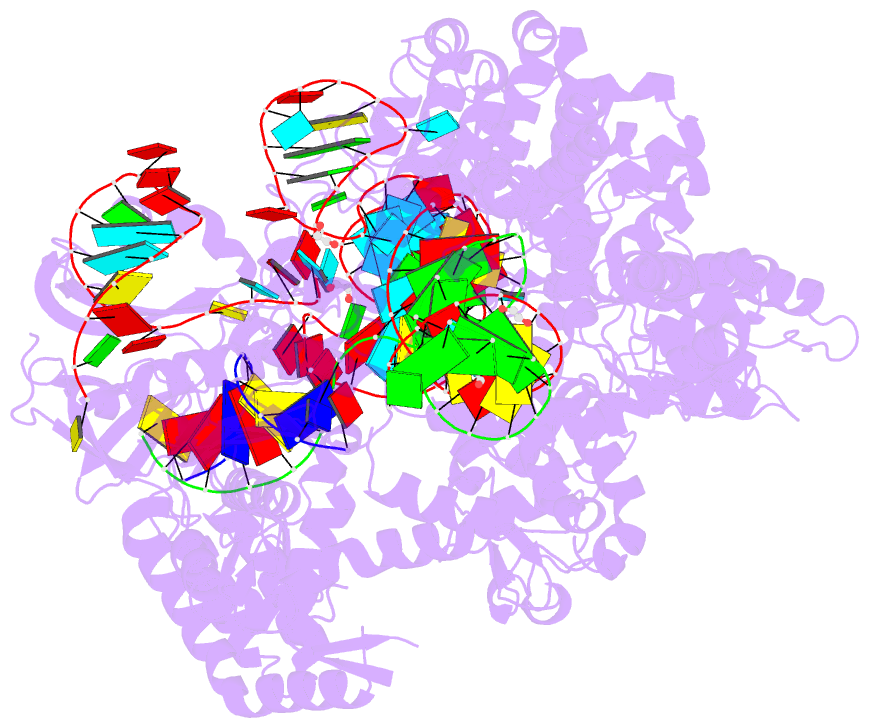
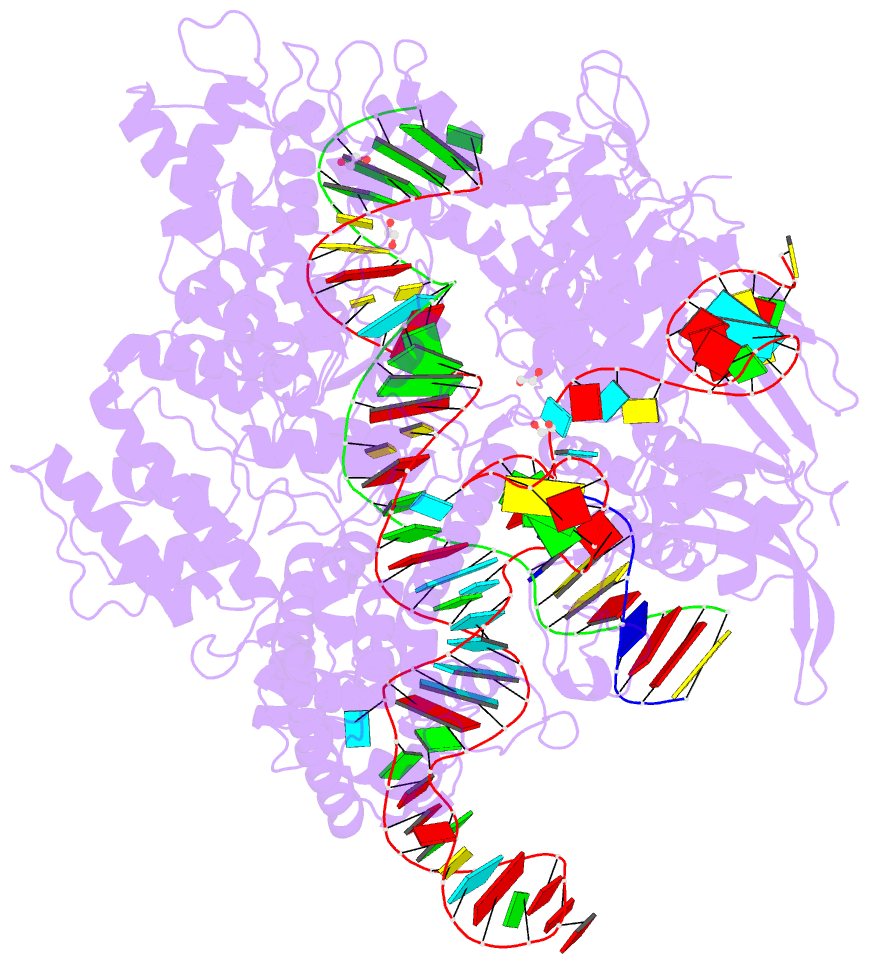
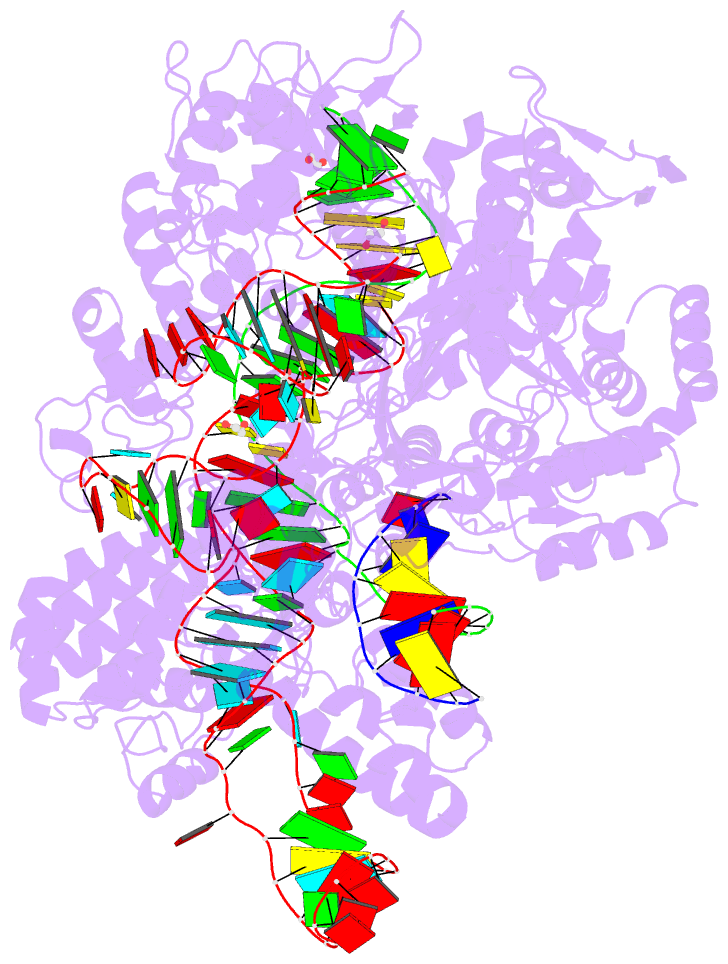
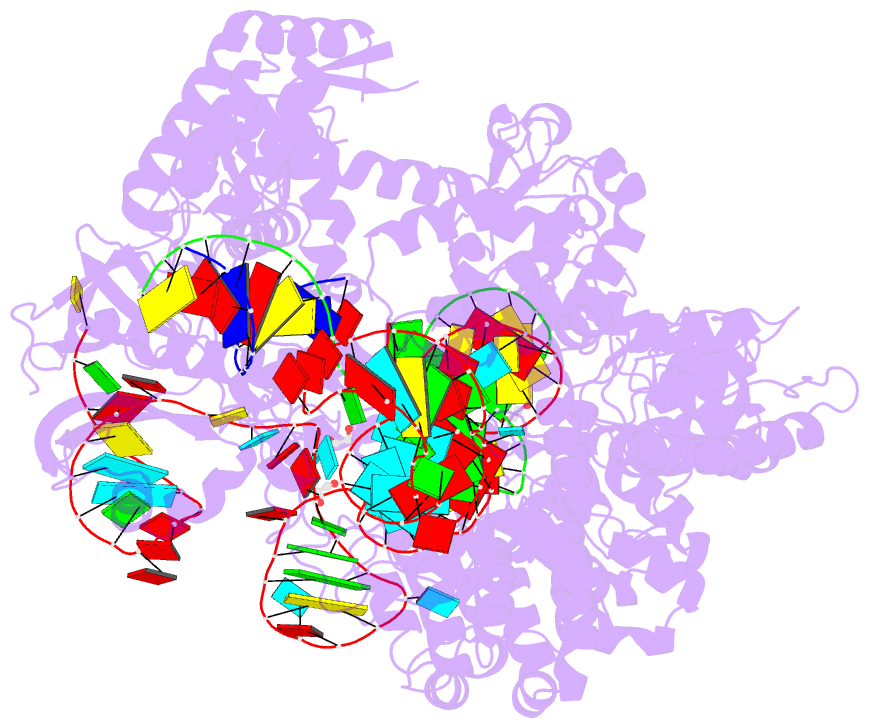
Summary information and primary citation
- PDB-id
- 7qqp; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase
- Method
- X-ray (2.6 Å)
- Summary
- Spcas9 bound to aavs1 off-target3 DNA substrate
- Reference
- Pacesa M, Lin CH, Clery A, Saha A, Arantes PR, Bargsten K, Irby MJ, Allain FH, Palermo G, Cameron P, Donohoue PD, Jinek M (2022): "Structural basis for Cas9 off-target activity." Cell, 185, 4067-4081.e21. doi: 10.1016/j.cell.2022.09.026.
- Abstract
- The target DNA specificity of the CRISPR-associated genome editor nuclease Cas9 is determined by complementarity to a 20-nucleotide segment in its guide RNA. However, Cas9 can bind and cleave partially complementary off-target sequences, which raises safety concerns for its use in clinical applications. Here, we report crystallographic structures of Cas9 bound to bona fide off-target substrates, revealing that off-target binding is enabled by a range of noncanonical base-pairing interactions within the guide:off-target heteroduplex. Off-target substrates containing single-nucleotide deletions relative to the guide RNA are accommodated by base skipping or multiple noncanonical base pairs rather than RNA bulge formation. Finally, PAM-distal mismatches result in duplex unpairing and induce a conformational change in the Cas9 REC lobe that perturbs its conformational activation. Together, these insights provide a structural rationale for the off-target activity of Cas9 and contribute to the improved rational design of guide RNAs and off-target prediction algorithms.