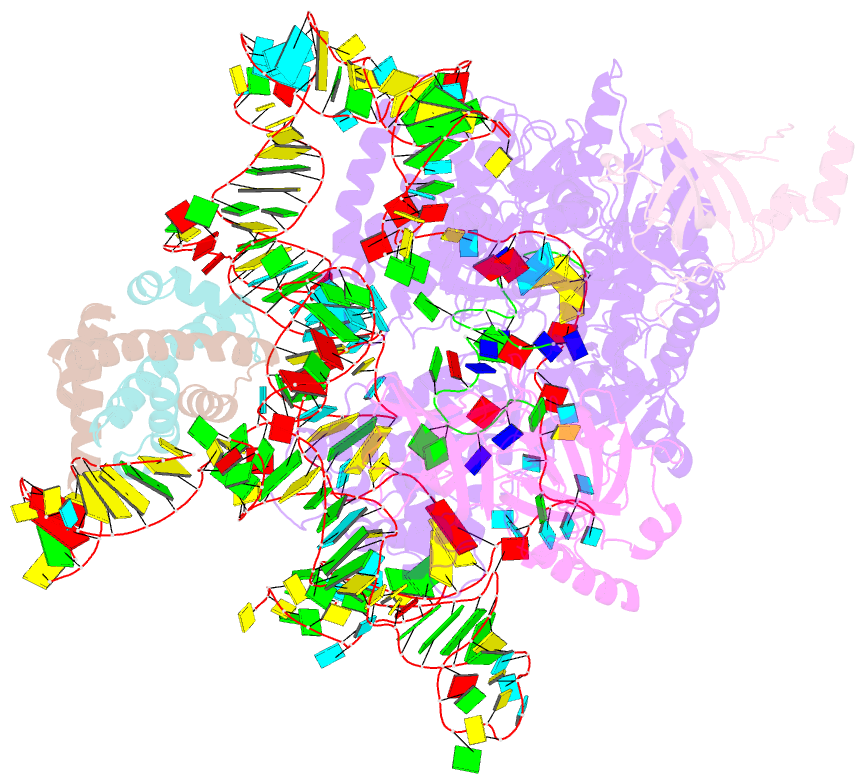
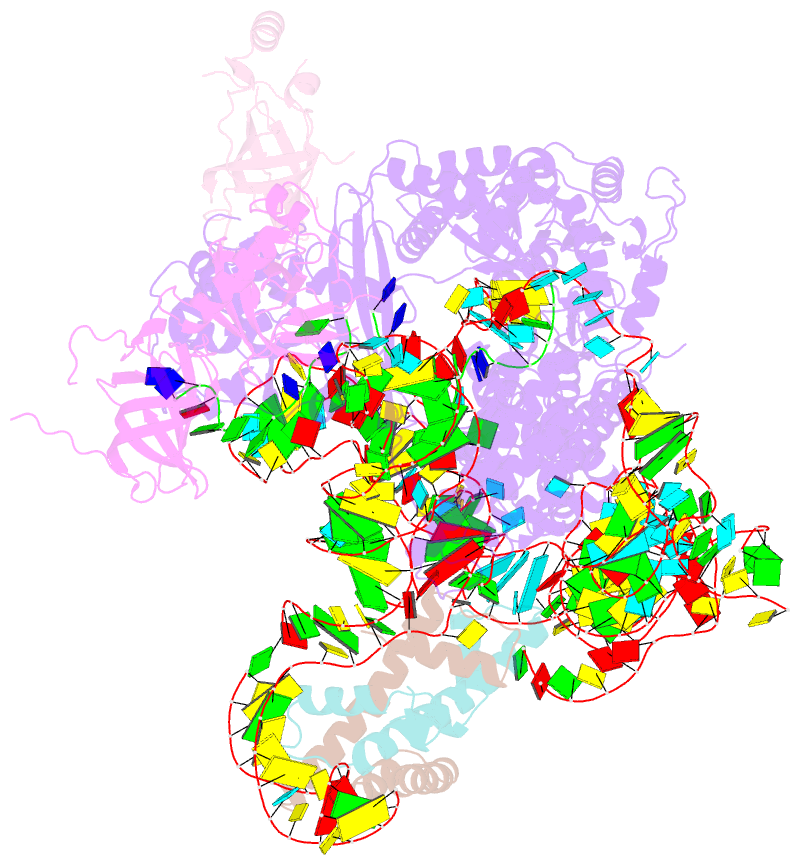
Summary information and primary citation
- PDB-id
- 7qxs; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein
- Method
- cryo-EM (3.9 Å)
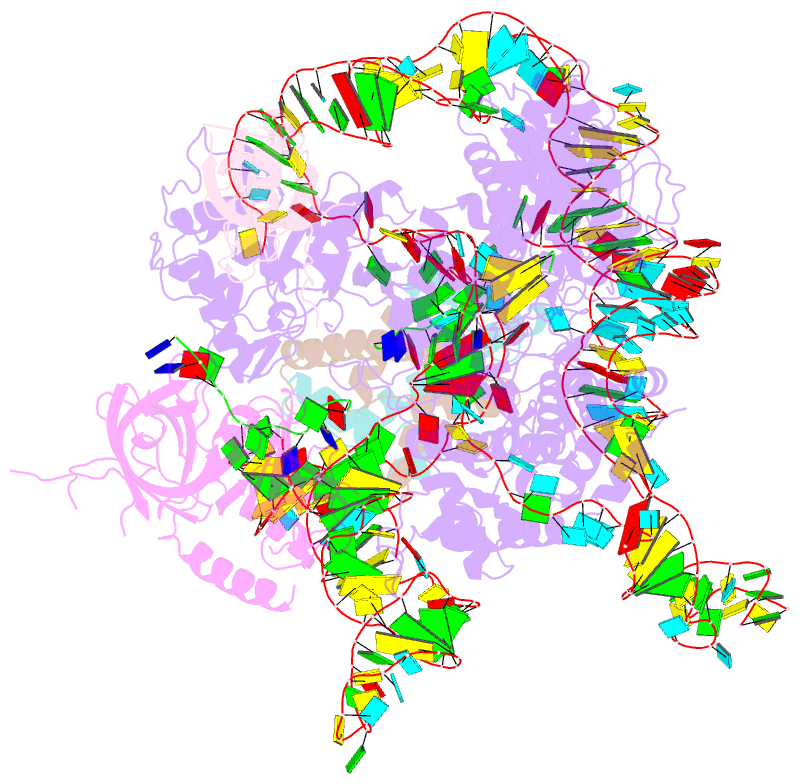
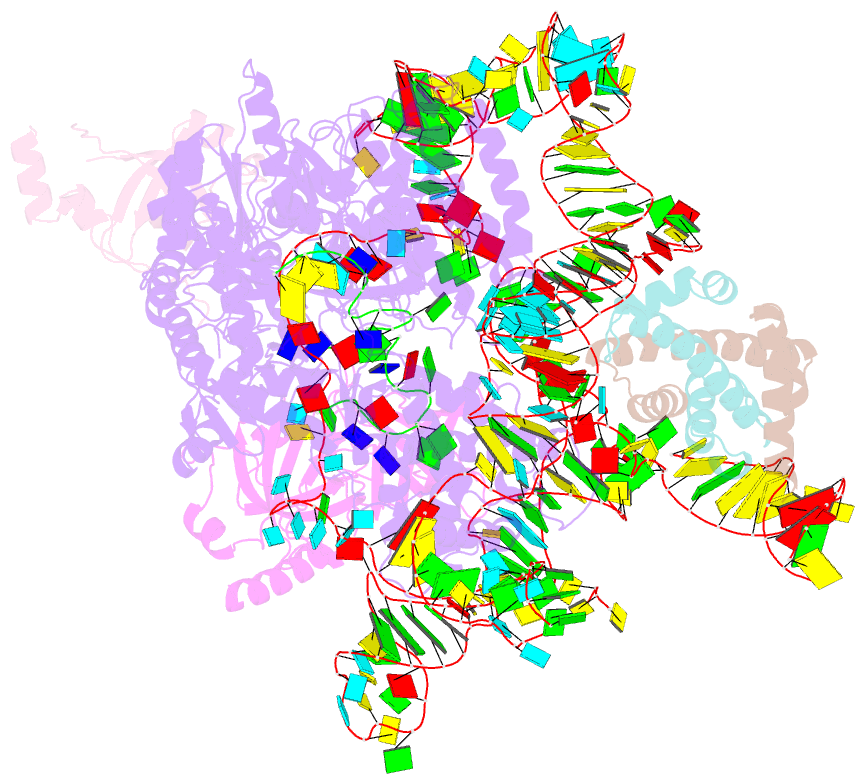
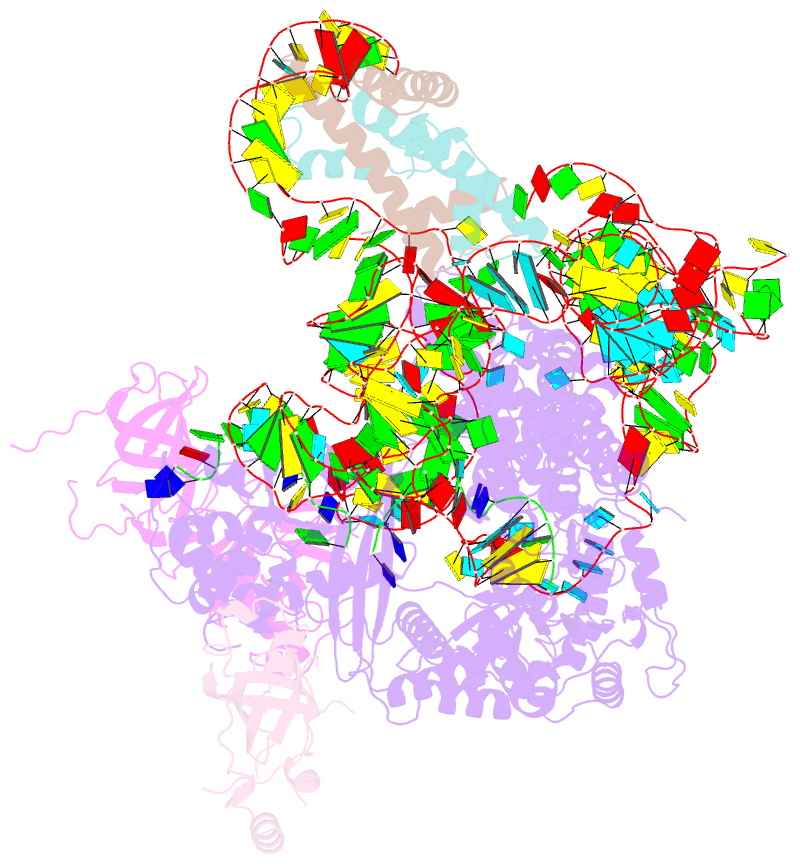
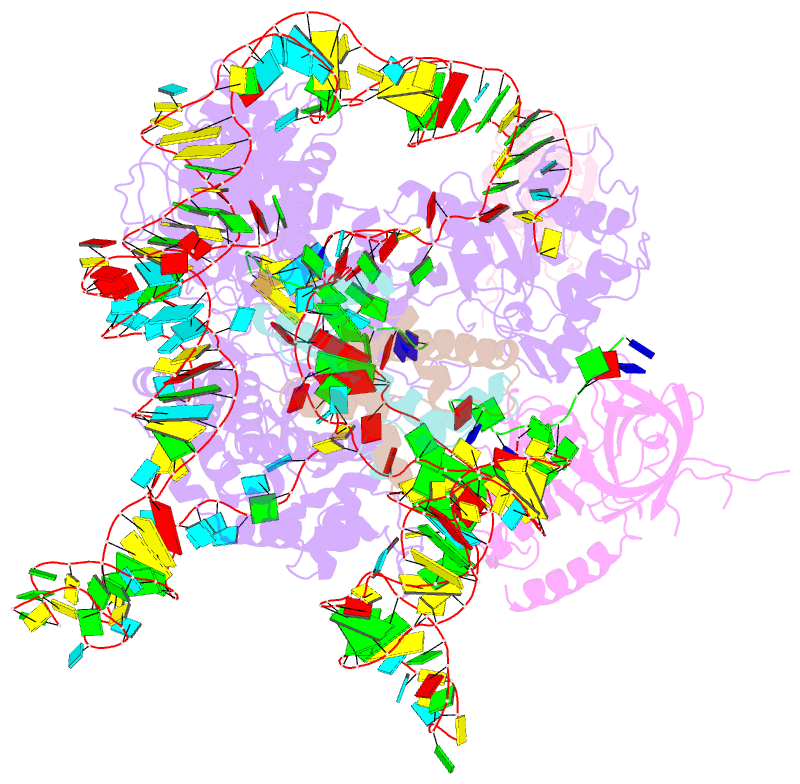
- Summary
- cryo-EM structure of human telomerase-DNA-tpp1-pot1 complex (with pot1 side chains)
- Reference
- Sekne Z, Ghanim GE, van Roon AM, Nguyen THD (2022): "Structural basis of human telomerase recruitment by TPP1-POT1." Science, 375, 1173-1176. doi: 10.1126/science.abn6840.
- Abstract
- Telomerase maintains genome stability by extending the 3' telomeric repeats at eukaryotic chromosome ends, thereby counterbalancing progressive loss caused by incomplete genome replication. In mammals, telomerase recruitment to telomeres is mediated by TPP1, which assembles as a heterodimer with POT1. We report structures of DNA-bound telomerase in complex with TPP1 and with TPP1-POT1 at 3.2- and 3.9-angstrom resolution, respectively. Our structures define interactions between telomerase and TPP1-POT1 that are crucial for telomerase recruitment to telomeres. The presence of TPP1-POT1 stabilizes the DNA, revealing an unexpected path by which DNA exits the telomerase active site and a DNA anchor site on telomerase that is important for telomerase processivity. Our findings rationalize extensive prior genetic and biochemical findings and provide a framework for future mechanistic work on telomerase regulation.