Summary information and primary citation
- PDB-id
- 7s0t; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- cryo-EM (3.05 Å)
- Summary
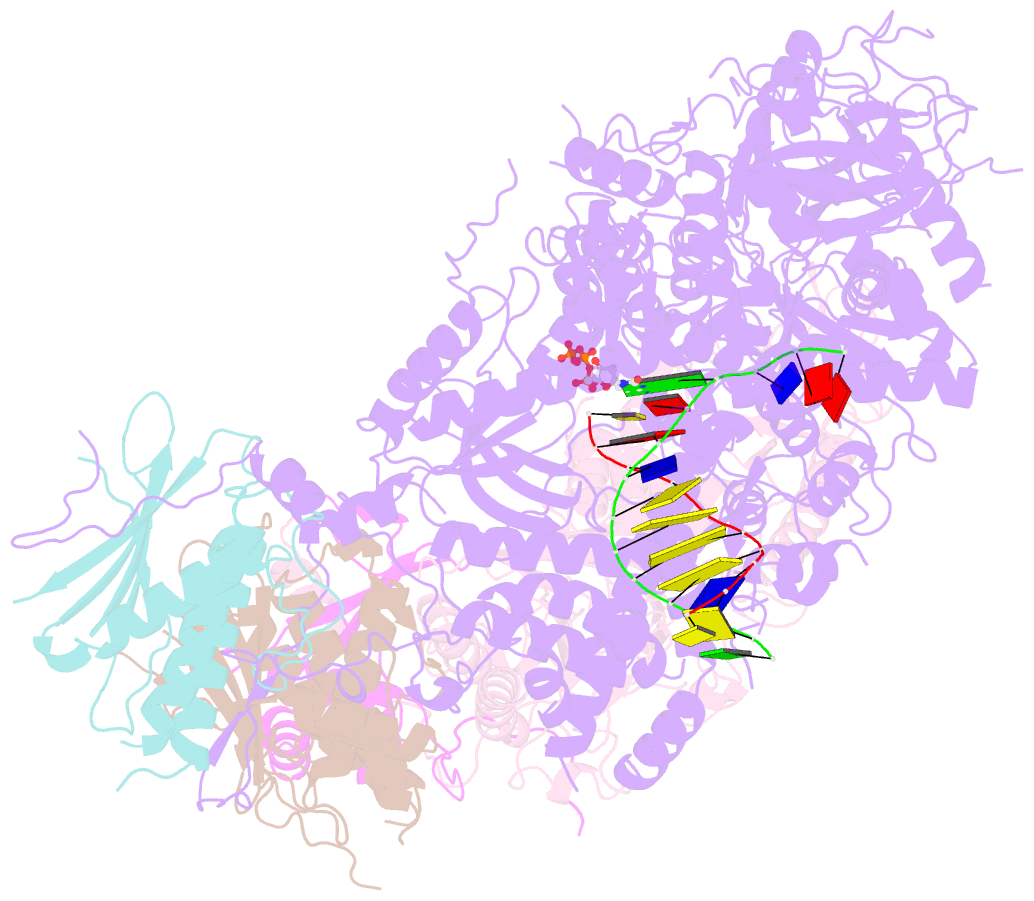
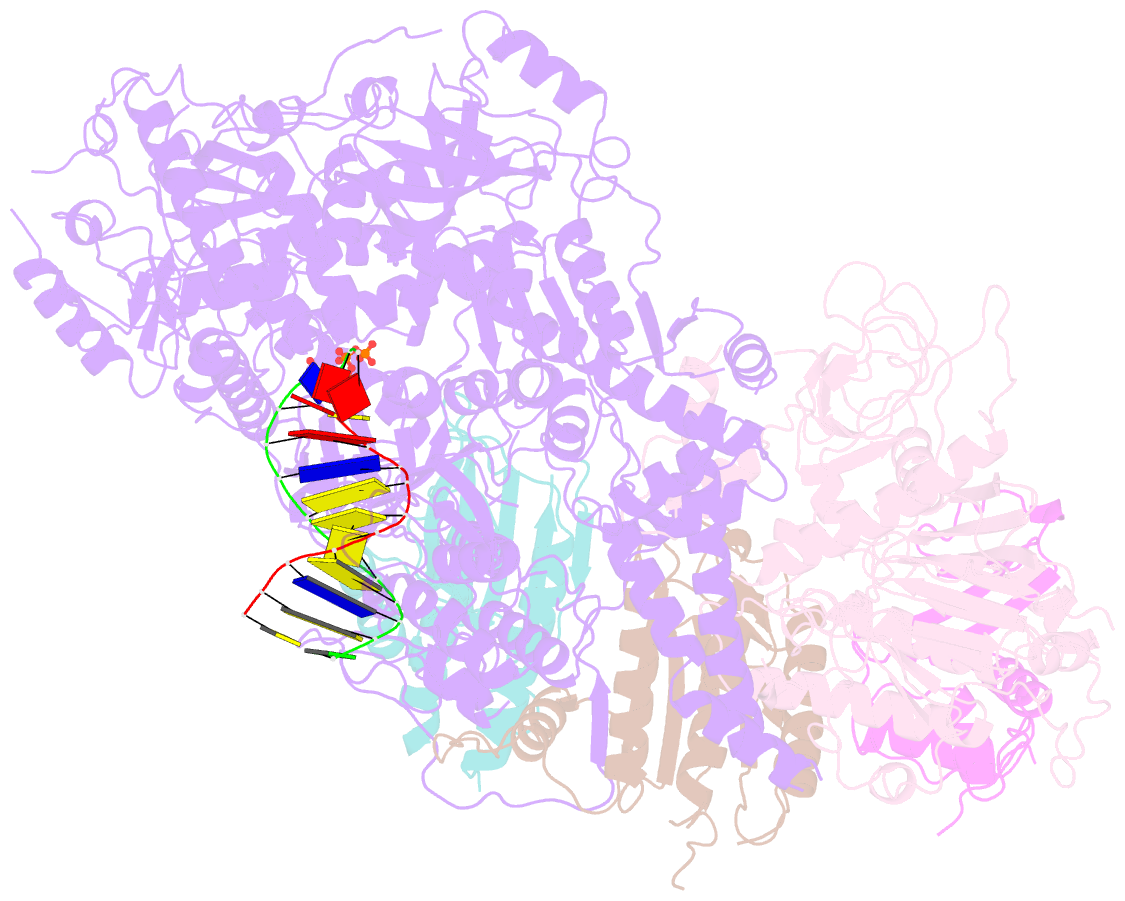
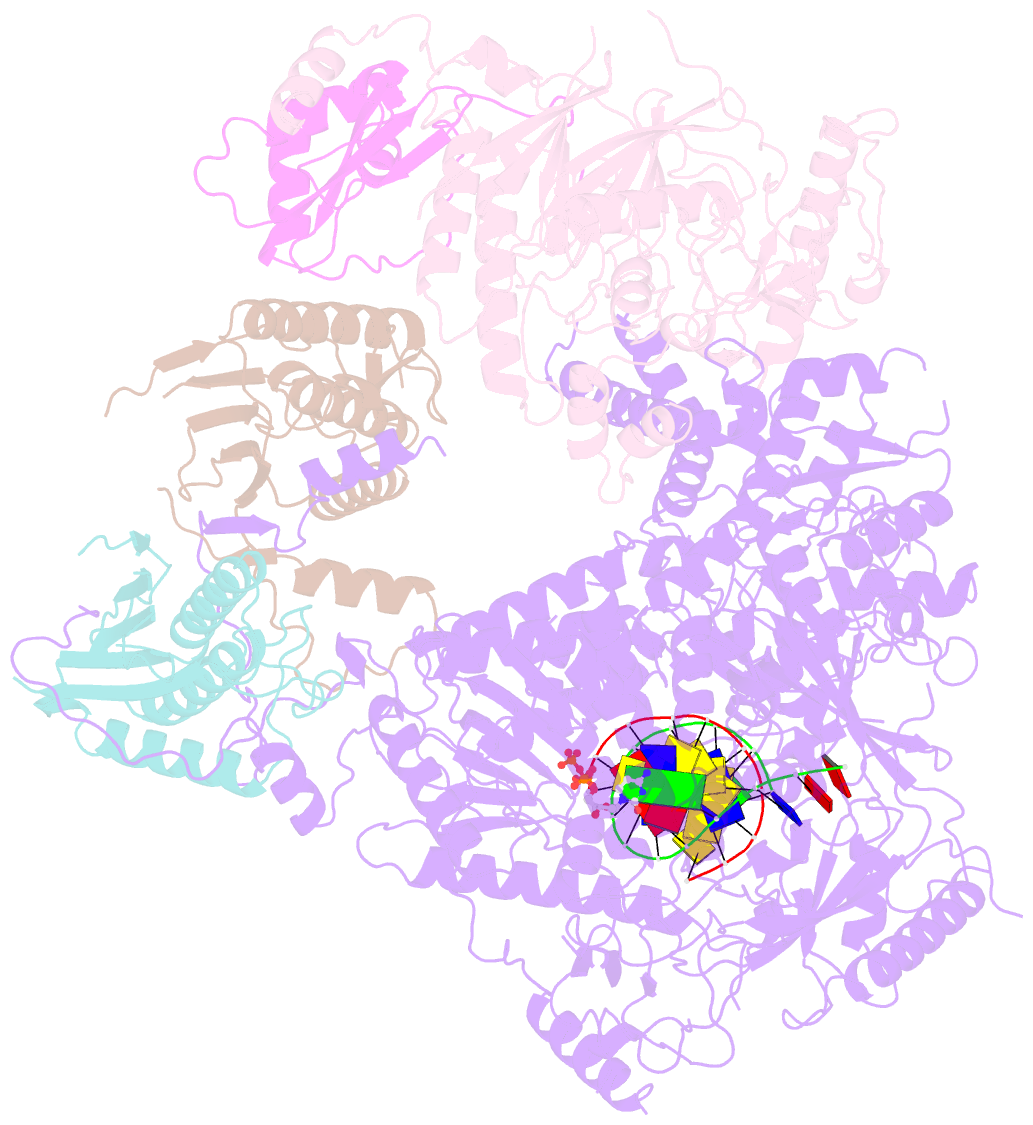
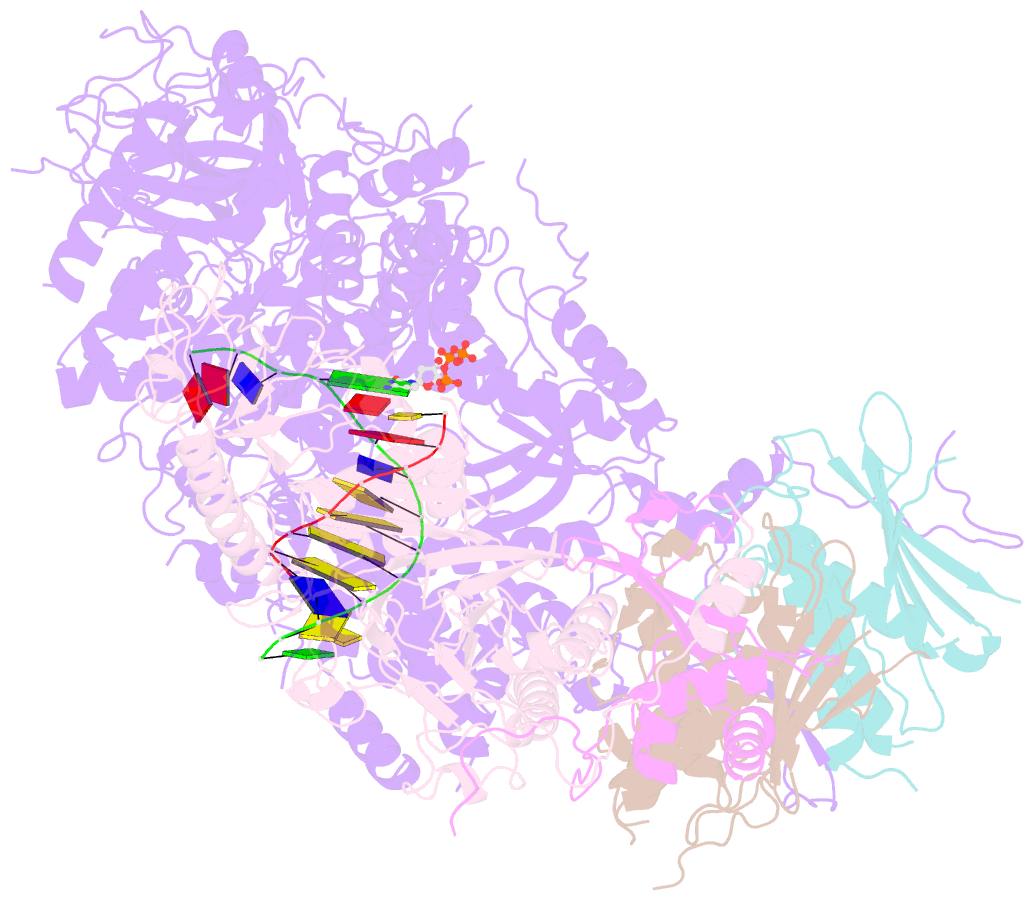
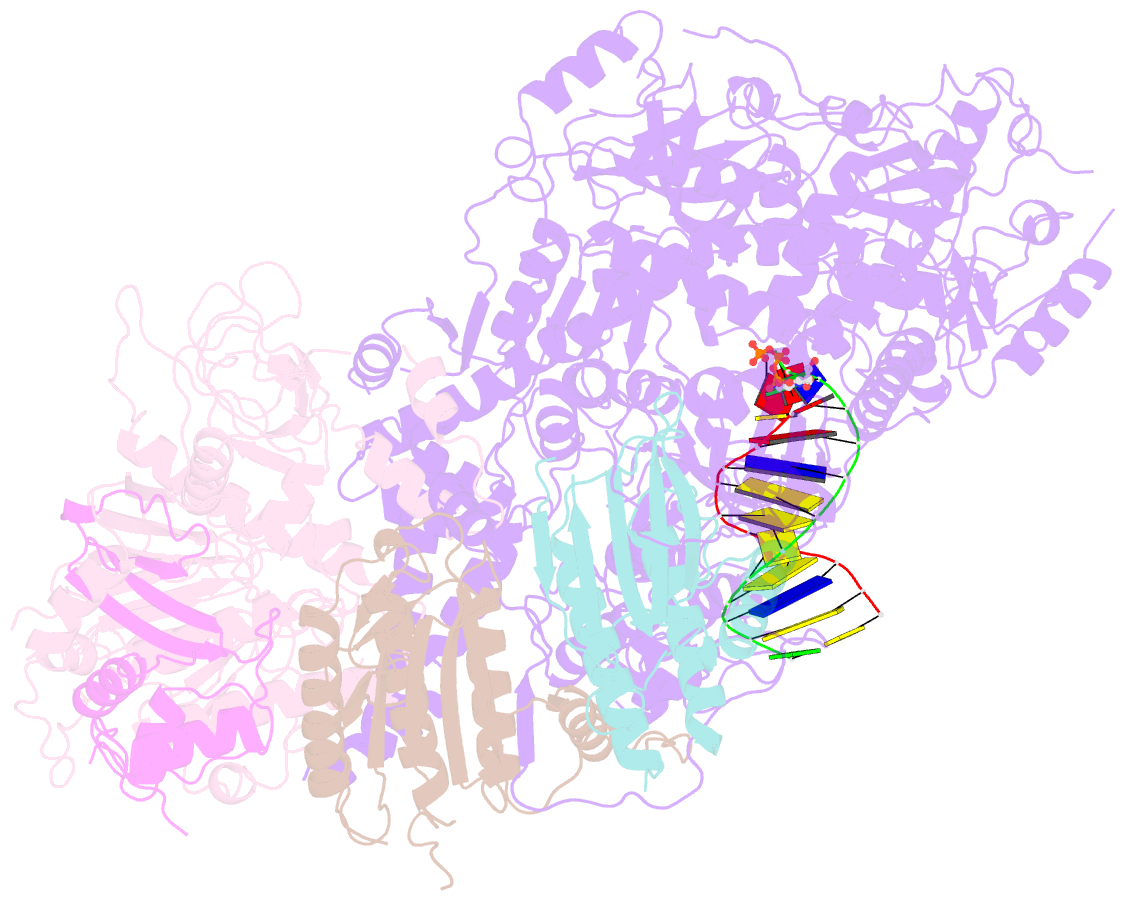
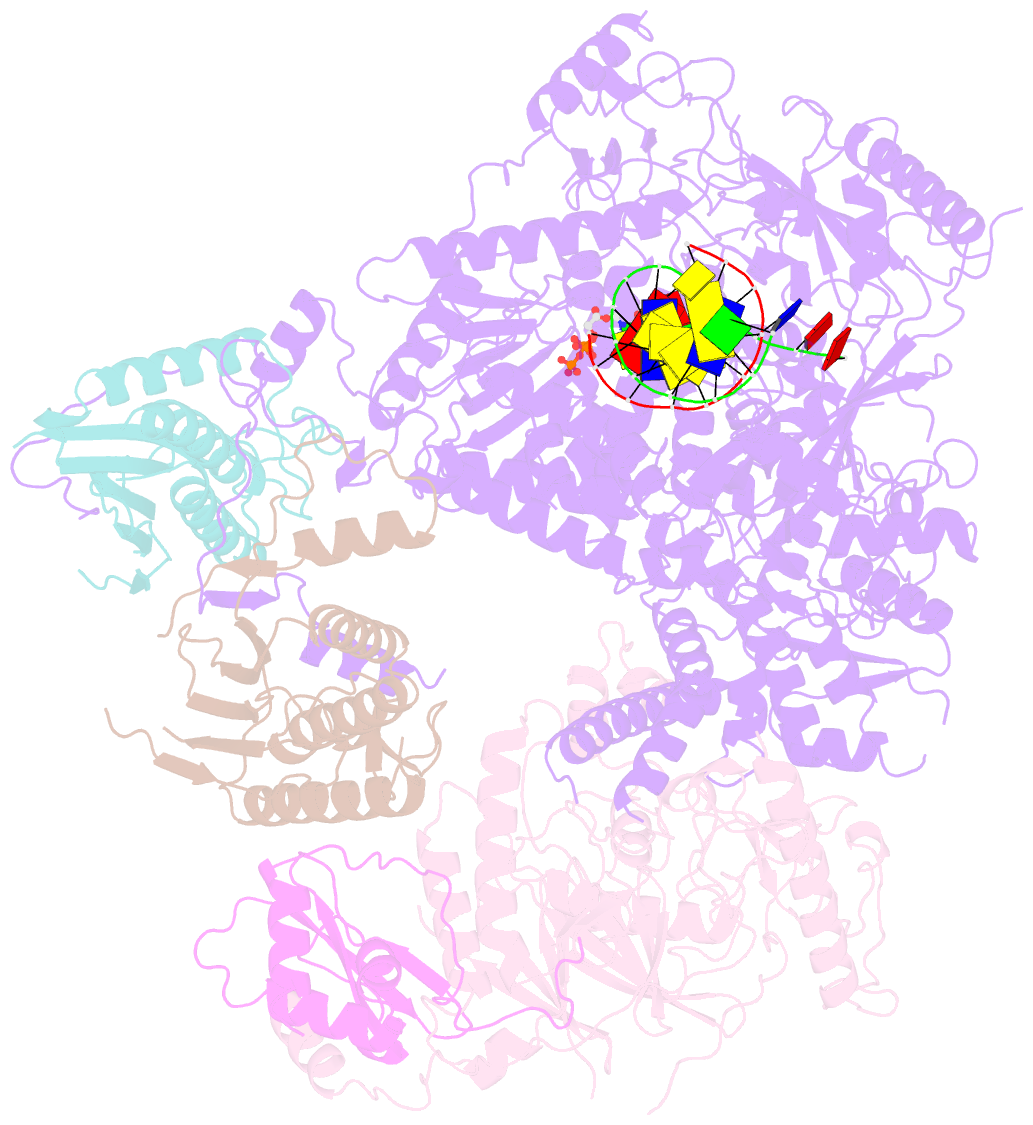
- Structure of DNA polymerase zeta with mismatched DNA
- Reference
- Malik R, Johnson RE, Prakash L, Prakash S, Ubarretxena-Belandia I, Aggarwal AK (2022): "Cryo-EM structure of translesion DNA synthesis polymerase zeta with a base pair mismatch." Nat Commun, 13, 1050. doi: 10.1038/s41467-022-28644-7.
- Abstract
- The B-family multi-subunit DNA polymerase ζ (Polζ) is important for translesion DNA synthesis (TLS) during replication, due to its ability to extend synthesis past nucleotides opposite DNA lesions and mismatched base pairs. We present a cryo-EM structure of Saccharomyces cerevisiae Polζ with an A:C mismatch at the primer terminus. The structure shows how the Polζ active site responds to the mismatched duplex DNA distortion, including the loosening of key protein-DNA interactions and a fingers domain in an "open" conformation, while the incoming dCTP is still able to bind for the extension reaction. The structure of the mismatched DNA-Polζ ternary complex reveals insights into mechanisms that either stall or favor continued DNA synthesis in eukaryotes.