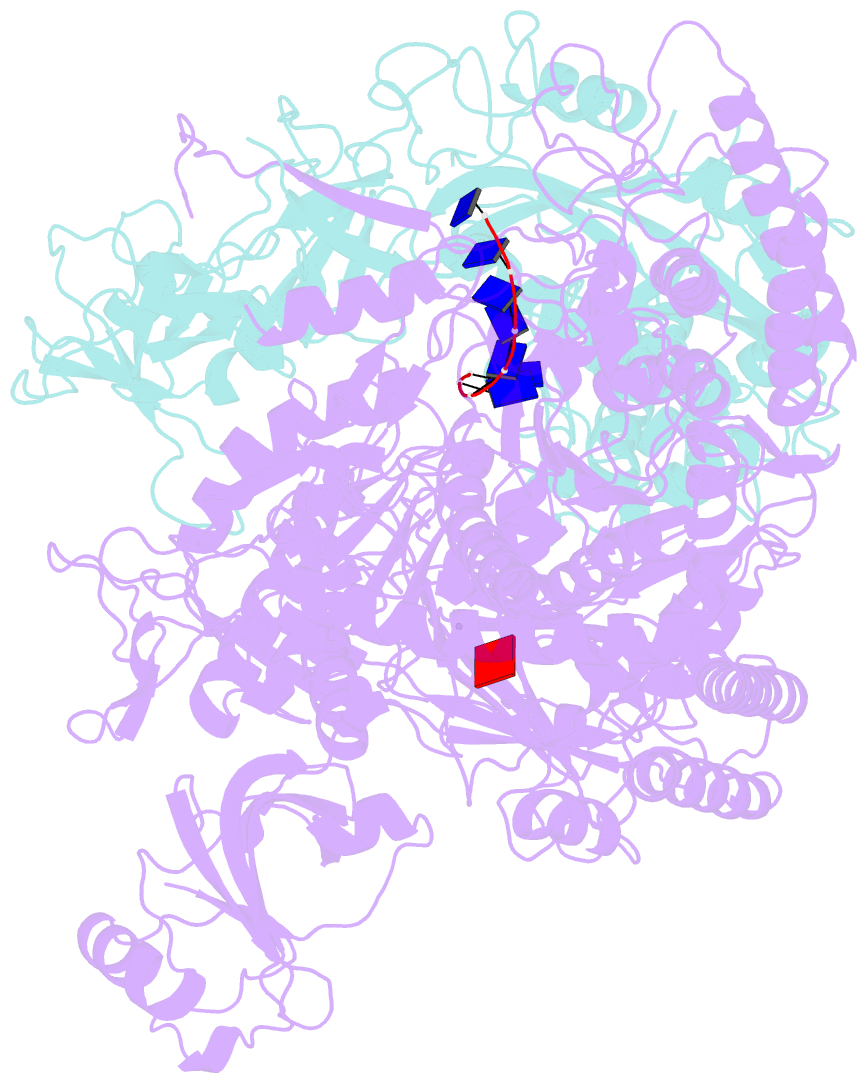
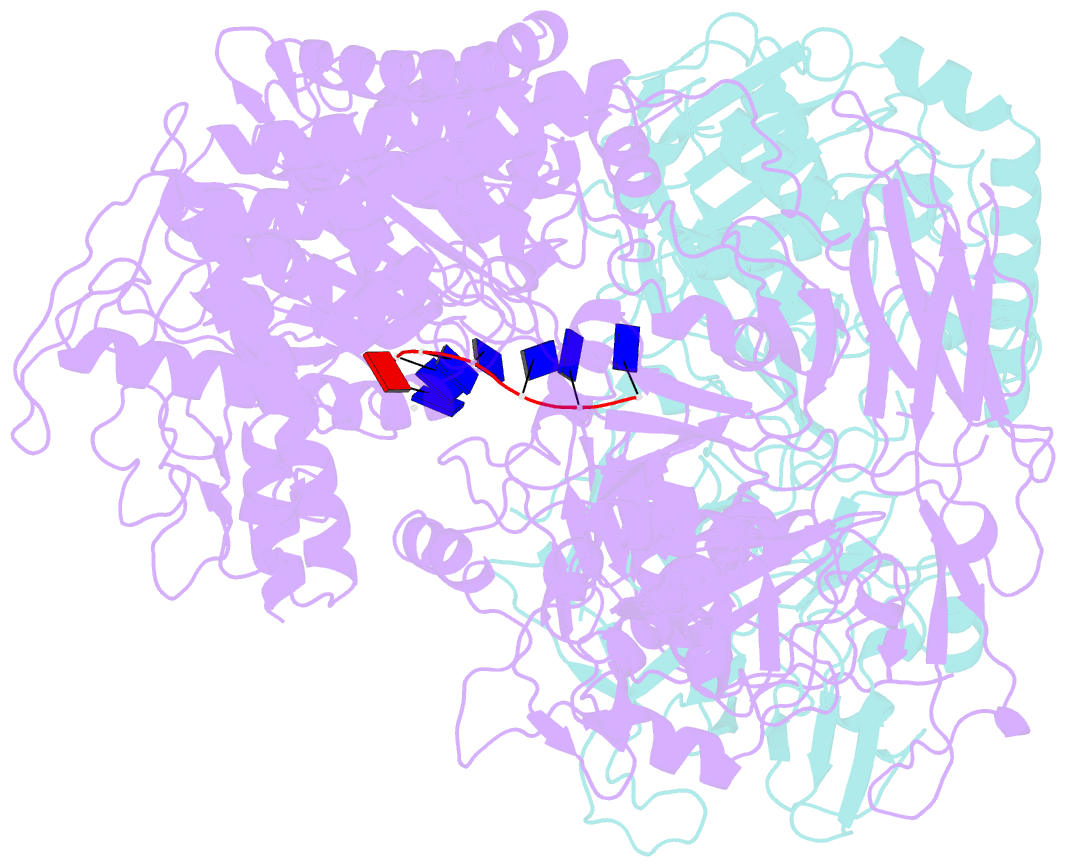
Summary information and primary citation
- PDB-id
- 7s9w; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- immune system
- Method
- cryo-EM (3.4 Å)
- Summary
- Structure of drmab:adp:DNA complex
- Reference
- Bravo JPK, Aparicio-Maldonado C, Nobrega FL, Brouns SJJ, Taylor DW (2022): "Structural basis for broad anti-phage immunity by DISARM." Nat Commun, 13, 2987. doi: 10.1038/s41467-022-30673-1.
- Abstract
- In the evolutionary arms race against phage, bacteria have assembled a diverse arsenal of antiviral immune strategies. While the recently discovered DISARM (Defense Island System Associated with Restriction-Modification) systems can provide protection against a wide range of phage, the molecular mechanisms that underpin broad antiviral targeting but avoiding autoimmunity remain enigmatic. Here, we report cryo-EM structures of the core DISARM complex, DrmAB, both alone and in complex with an unmethylated phage DNA mimetic. These structures reveal that DrmAB core complex is autoinhibited by a trigger loop (TL) within DrmA and binding to DNA substrates containing a 5' overhang dislodges the TL, initiating a long-range structural rearrangement for DrmAB activation. Together with structure-guided in vivo studies, our work provides insights into the mechanism of phage DNA recognition and specific activation of this widespread antiviral defense system.