Summary information and primary citation
- PDB-id
- 7tqq; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (2.2 Å)
- Summary
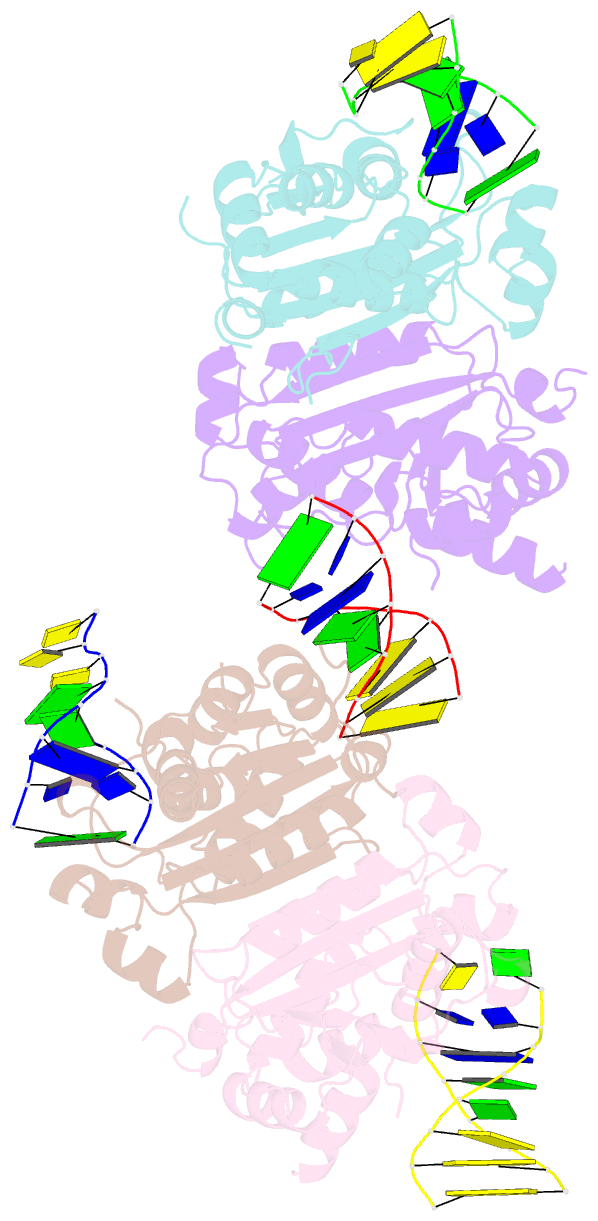
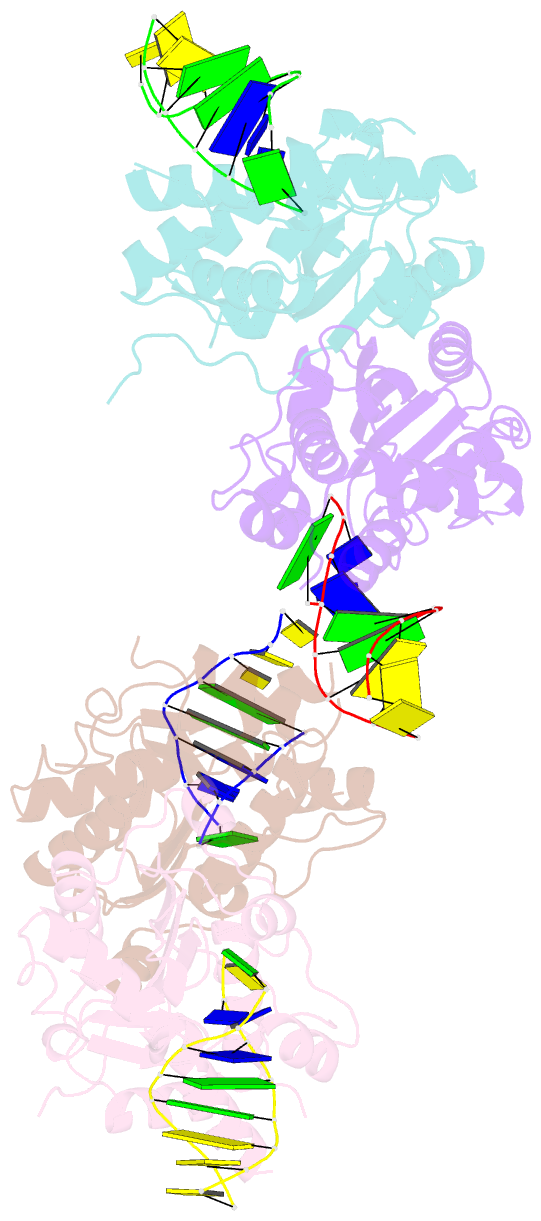
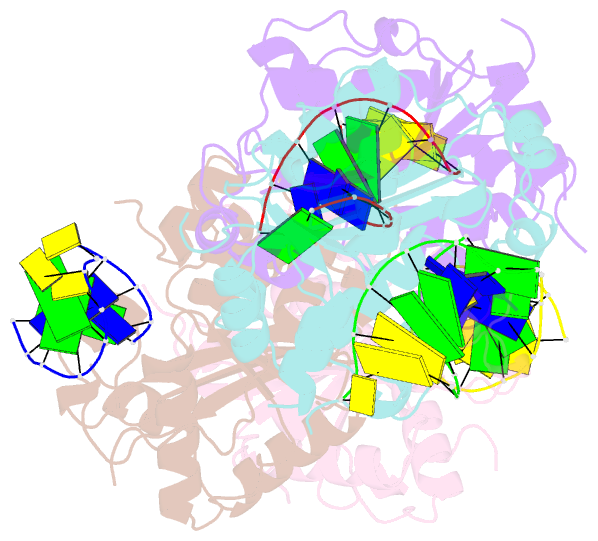
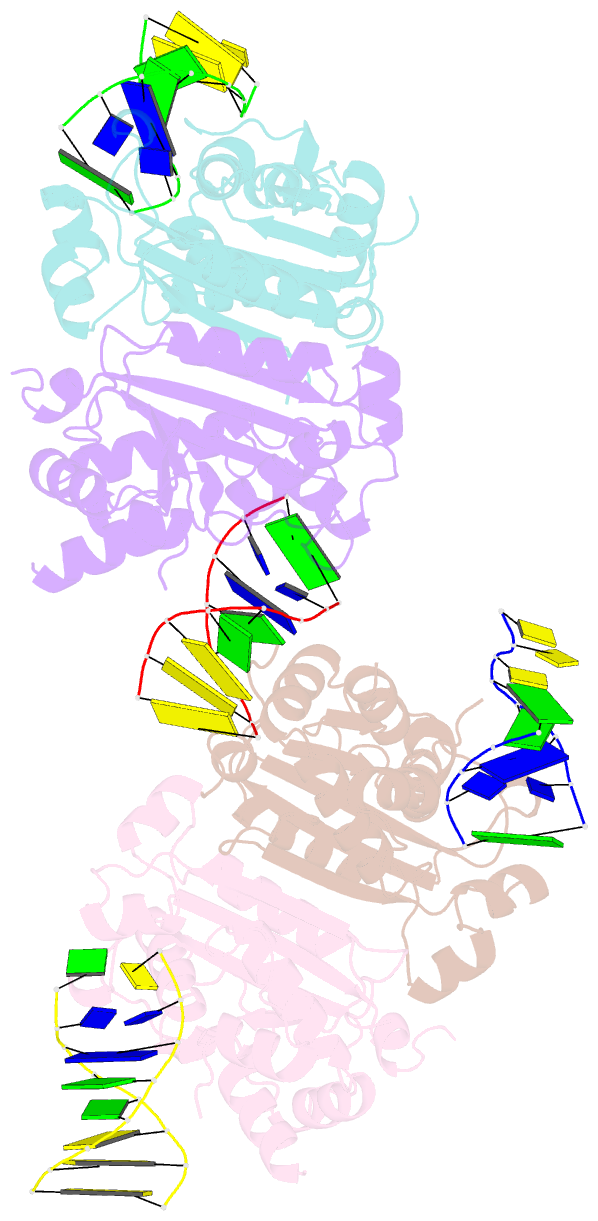
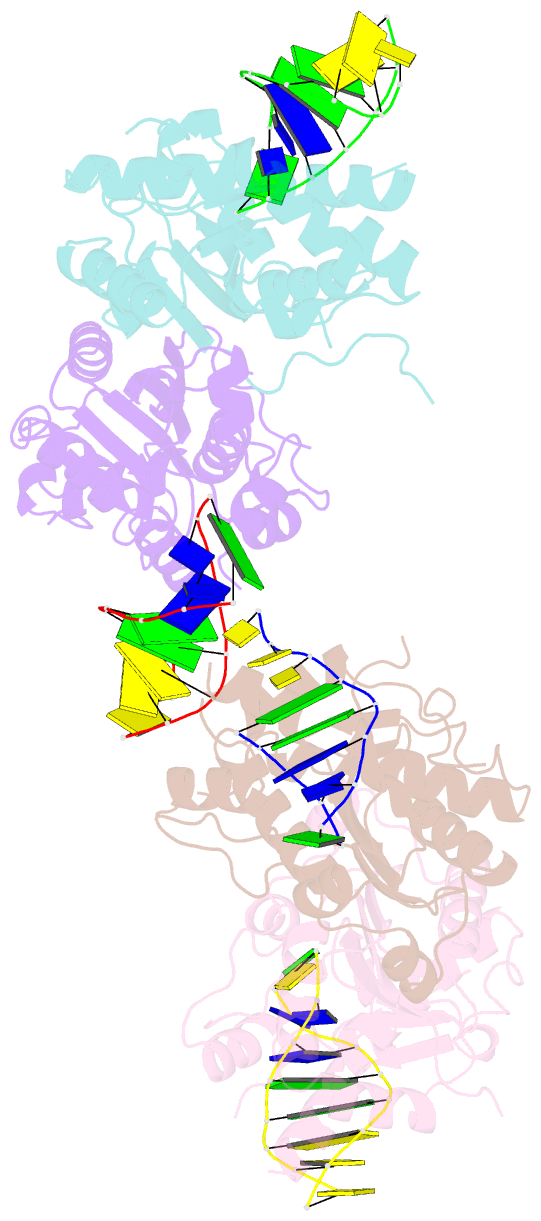
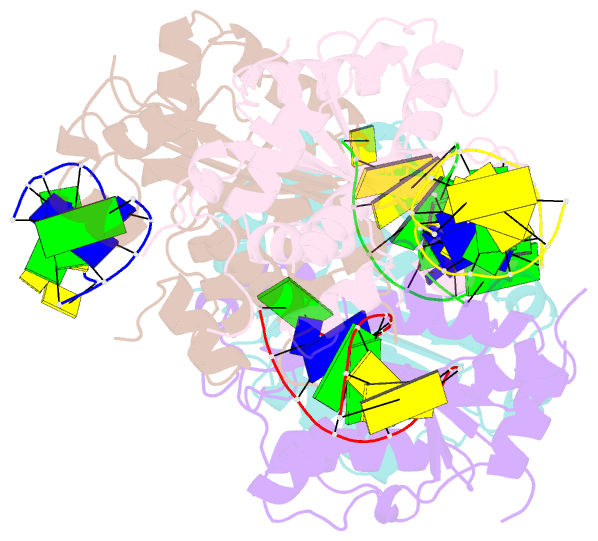
- Structure of human trex1-DNA complex
- Reference
- Zhou W, Richmond-Buccola D, Wang Q, Kranzusch PJ (2022): "Structural basis of human TREX1 DNA degradation and autoimmune disease." Nat Commun, 13, 4277. doi: 10.1038/s41467-022-32055-z.
- Abstract
- TREX1 is a cytosolic DNA nuclease essential for regulation of cGAS-STING immune signaling. Existing structures of mouse TREX1 establish a mechanism of DNA degradation and provide a key model to explain autoimmune disease, but these structures incompletely explain human disease-associated mutations and have limited ability to guide development of small-molecule therapeutics. Here we determine crystal structures of human TREX1 in apo and DNA-bound conformations that provide high-resolution detail of all human-specific features. A 1.25 Å structure of human TREX1 establishes a complete model of solvation of the exonuclease active site and a 2.2 Å structure of the human TREX1-DNA complex enables identification of specific substitutions involved in DNA recognition. We map each TREX1 mutation associated with autoimmune disease and establish distinct categories of substitutions predicted to impact enzymatic function, protein stability, and interaction with cGAS-DNA liquid droplets. Our results explain how human-specific substitutions regulate TREX1 function and provide a foundation for structure-guided design of TREX1 therapeutics.