Summary information and primary citation
- PDB-id
- 7u2a; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ligase-RNA
- Method
- cryo-EM (4.1 Å)
- Summary
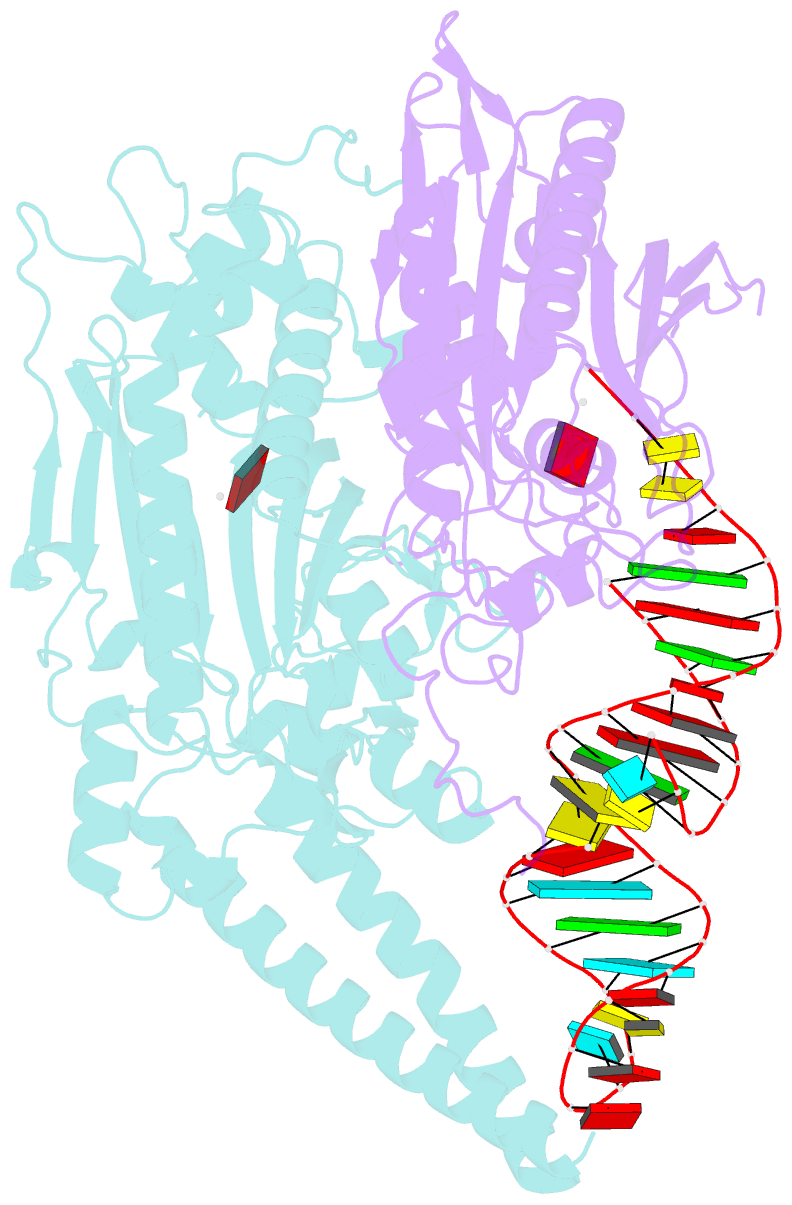
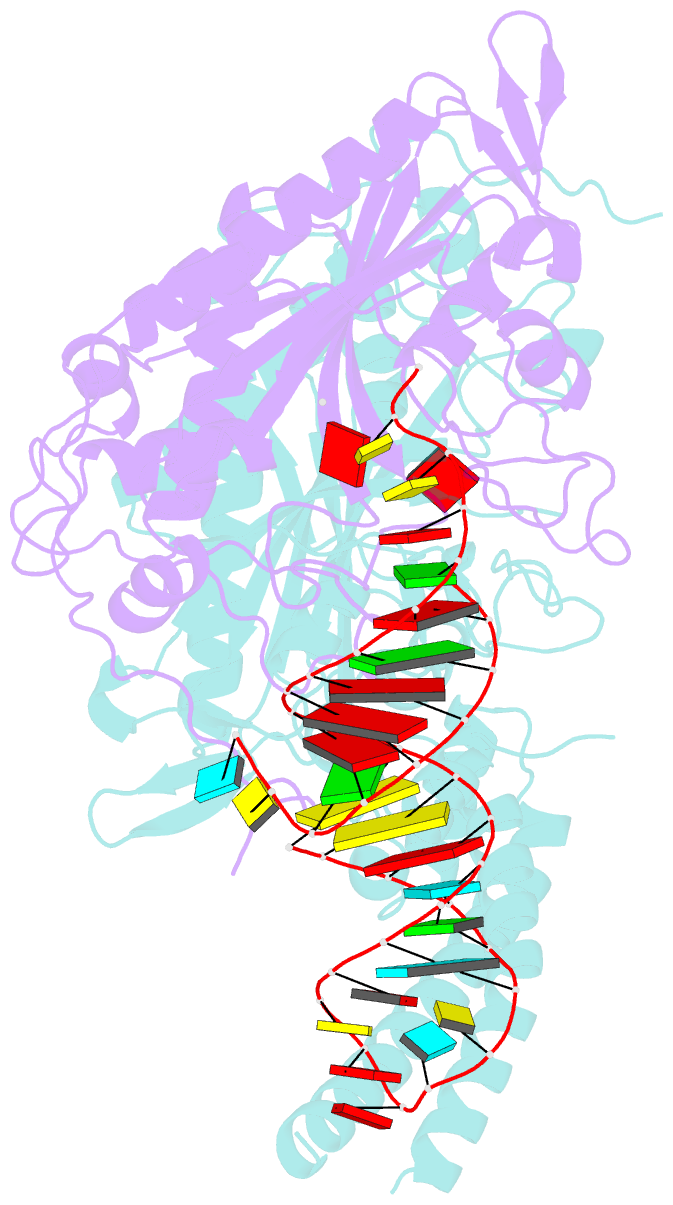
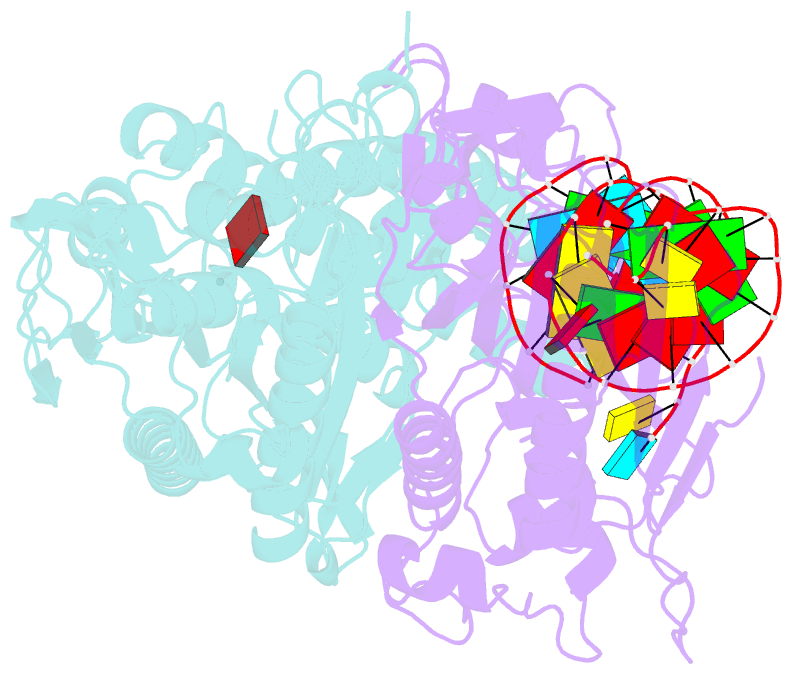
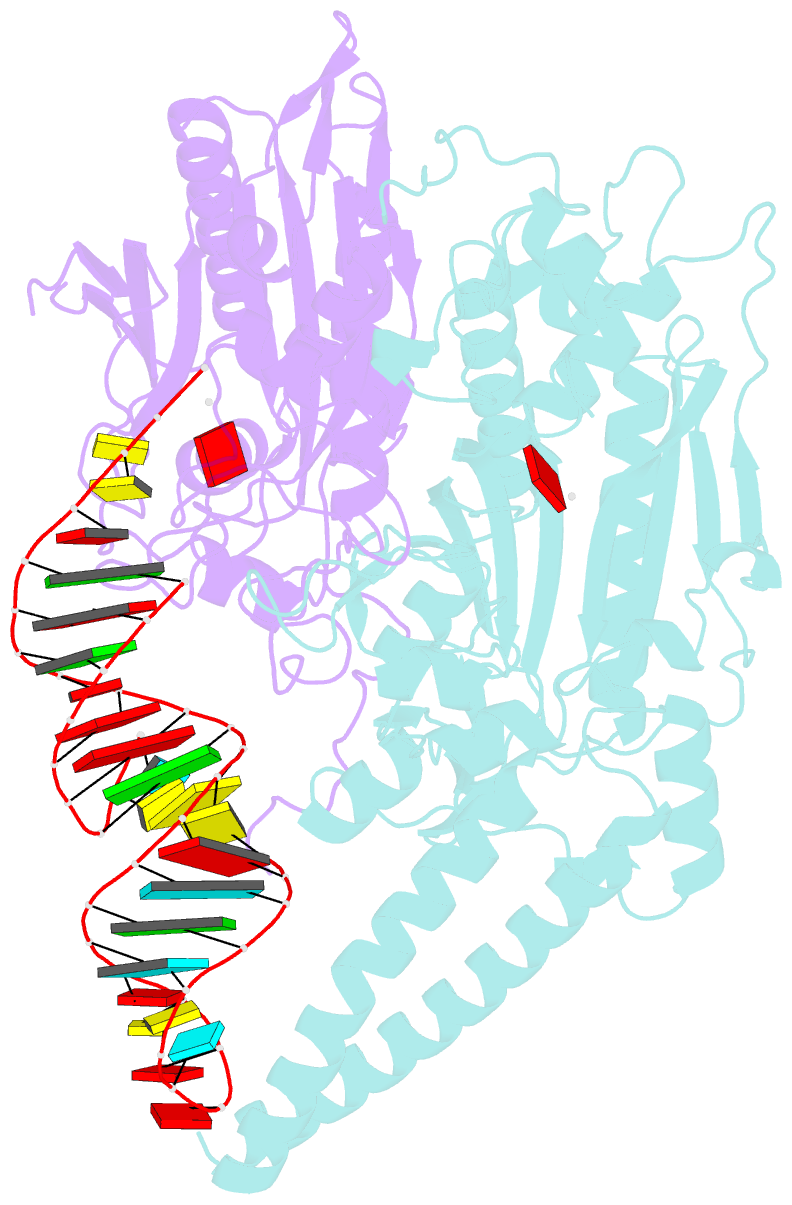
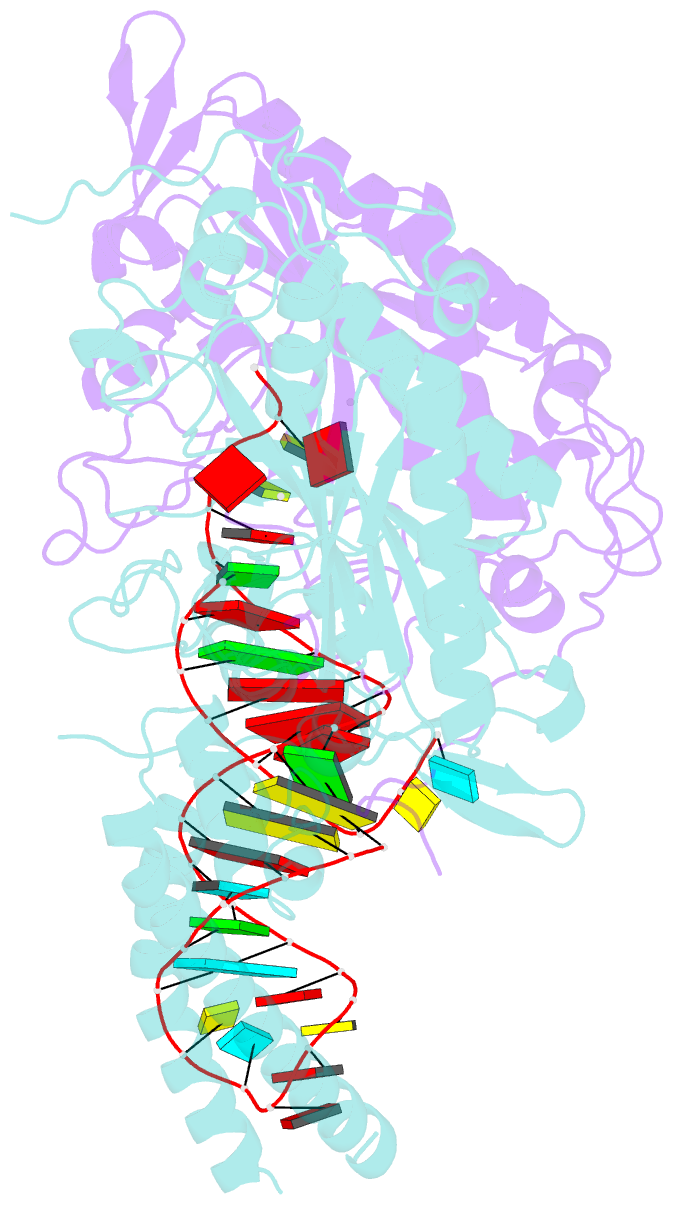
- Cryo-electron microscopy structure of human mt-serrs in complex with mt-trna (gcu)
- Reference
- Kuhle B, Hirschi M, Doerfel LK, Lander GC, Schimmel P (2022): "Structural basis for shape-selective recognition and aminoacylation of a D-armless human mitochondrial tRNA." Nat Commun, 13, 5100. doi: 10.1038/s41467-022-32544-1.
- Abstract
- Human mitochondrial gene expression relies on the specific recognition and aminoacylation of mitochondrial tRNAs (mtRNAs) by nuclear-encoded mitochondrial aminoacyl-tRNA synthetases (mt-aaRSs). Despite their essential role in cellular energy homeostasis, strong mutation pressure and genetic drift have led to an unparalleled sequence erosion of animal mtRNAs. The structural and functional consequences of this erosion are not understood. Here, we present cryo-EM structures of the human mitochondrial seryl-tRNA synthetase (mSerRS) in complex with mtRNASer(GCU). These structures reveal a unique mechanism of substrate recognition and aminoacylation. The mtRNASer(GCU) is highly degenerated, having lost the entire D-arm, tertiary core, and stable L-shaped fold that define canonical tRNAs. Instead, mtRNASer(GCU) evolved unique structural innovations, including a radically altered T-arm topology that serves as critical identity determinant in an unusual shape-selective readout mechanism by mSerRS. Our results provide a molecular framework to understand the principles of mito-nuclear co-evolution and specialized mechanisms of tRNA recognition in mammalian mitochondrial gene expression.