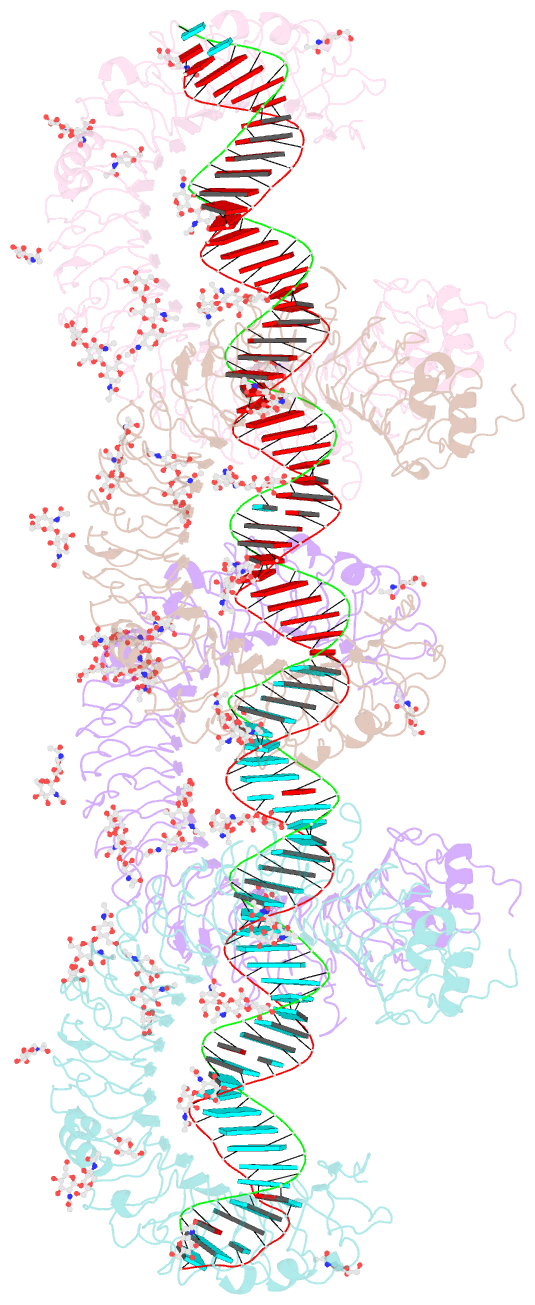
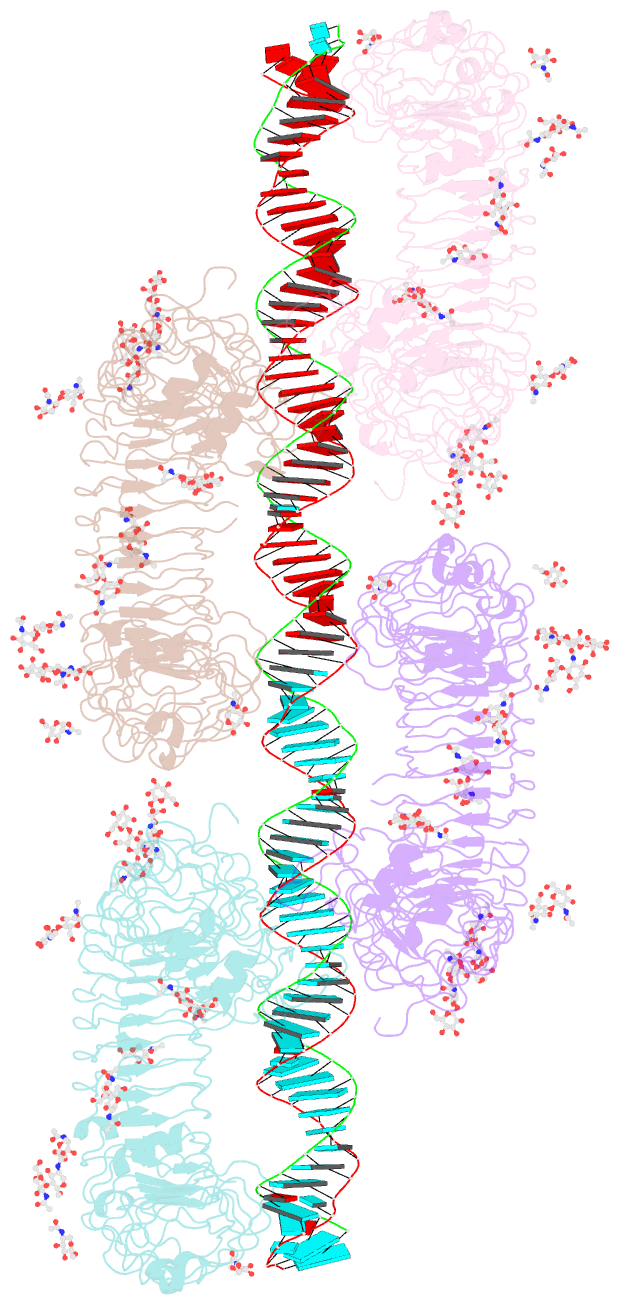
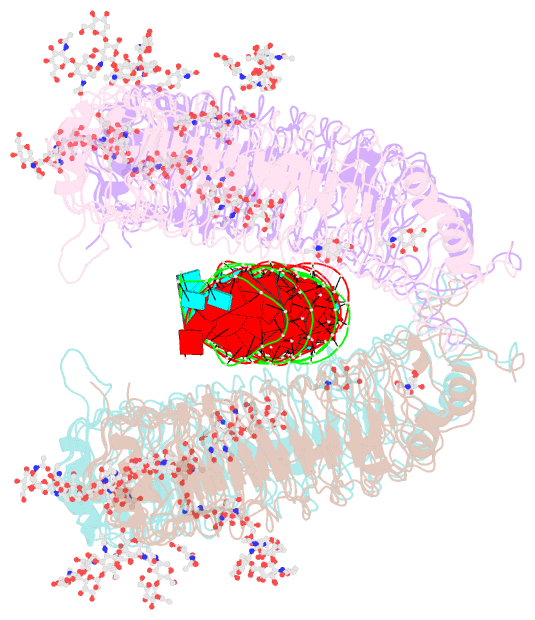
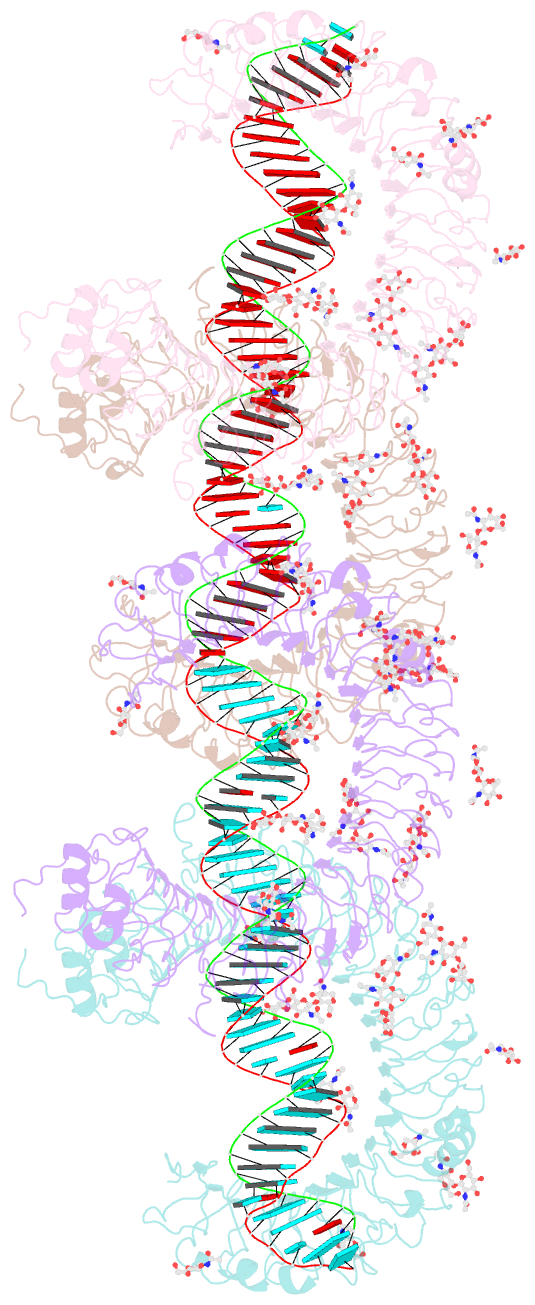
Summary information and primary citation
- PDB-id
- 7wm4; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- immune system-RNA
- Method
- cryo-EM (3.2 Å)
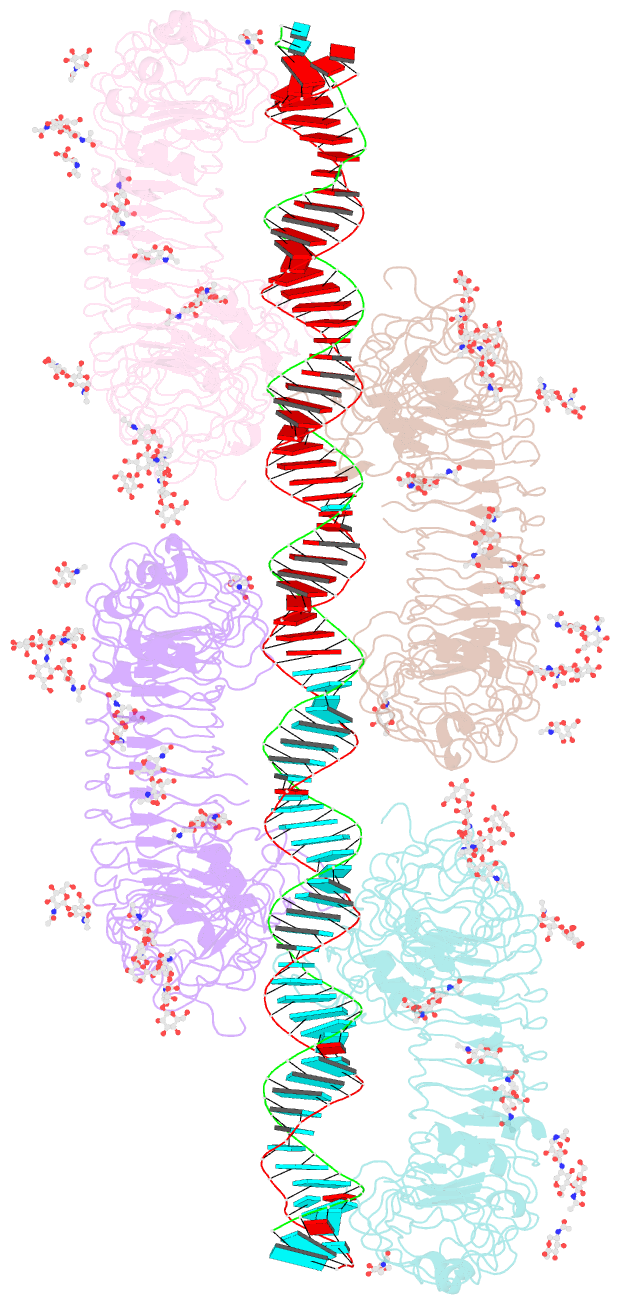
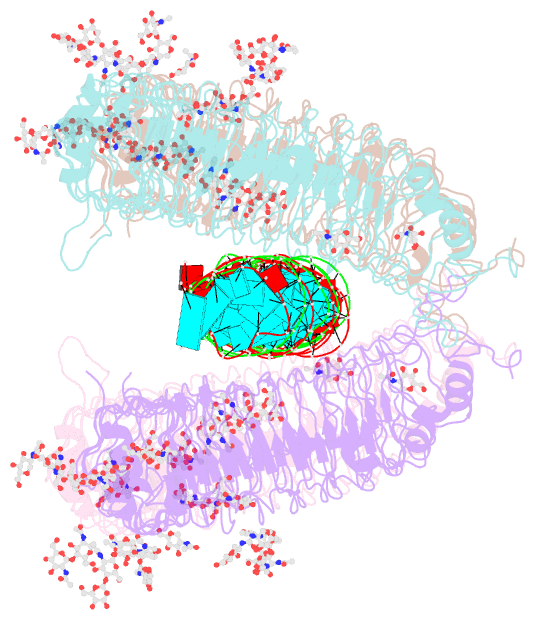
- Summary
- cryo-EM structure of tetrameric tlr3 in complex with dsrna (90 bp)
- Reference
- Sakaniwa K, Fujimura A, Shibata T, Shigematsu H, Ekimoto T, Yamamoto M, Ikeguchi M, Miyake K, Ohto U, Shimizu T (2023): "TLR3 forms a laterally aligned multimeric complex along double-stranded RNA for efficient signal transduction." Nat Commun, 14, 164. doi: 10.1038/s41467-023-35844-2.
- Abstract
- Toll-like receptor 3 (TLR3) is a member of the TLR family, which plays an important role in the innate immune system and is responsible for recognizing viral double-stranded RNA (dsRNA). Previous biochemical and structural studies have revealed that a minimum length of approximately 40-50 base pairs of dsRNA is necessary for TLR3 binding and dimerization. However, efficient TLR3 activation requires longer dsRNA and the molecular mechanism underlying its dsRNA length-dependent activation remains unknown. Here, we report cryo-electron microscopy analyses of TLR3 complexed with longer dsRNA. TLR3 dimers laterally form a higher multimeric complex along dsRNA, providing the basis for cooperative binding and efficient signal transduction.