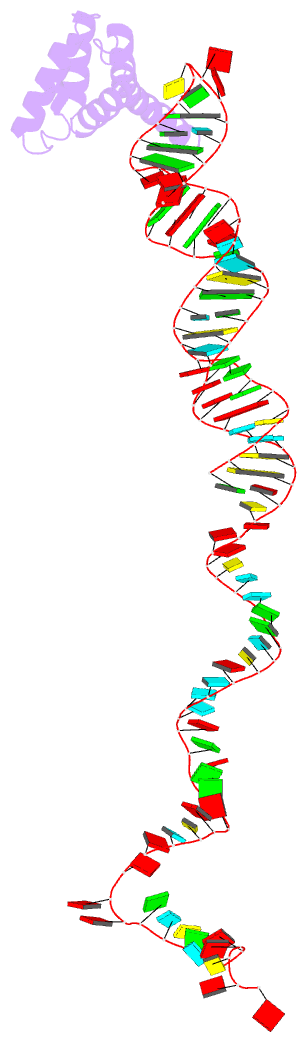
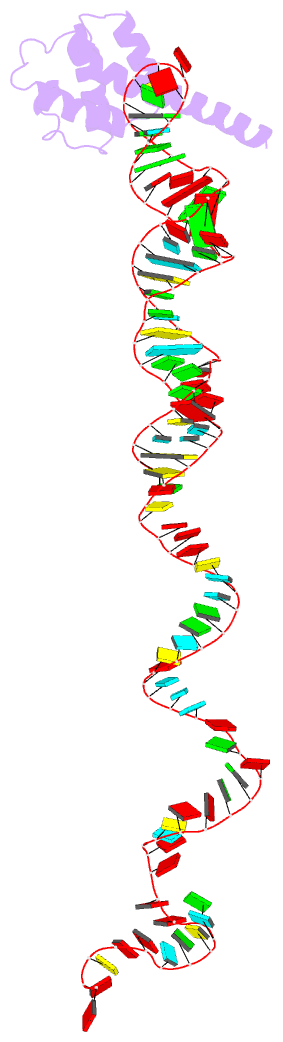
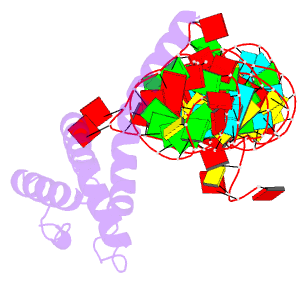
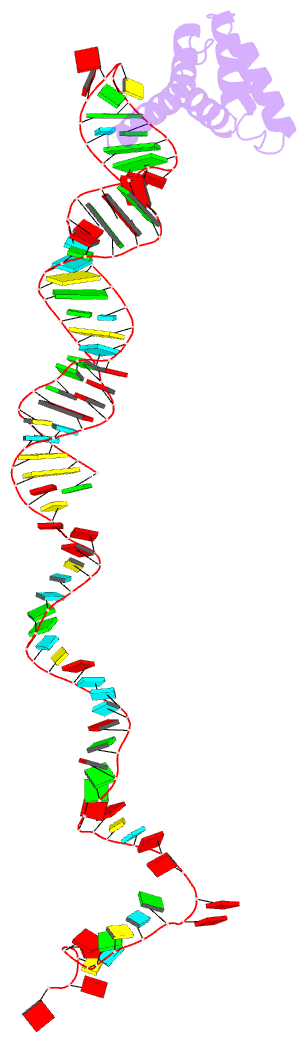
Summary information and primary citation
- PDB-id
- 7x34; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- translation-RNA
- Method
- cryo-EM (3.1 Å)
- Summary
- cryo-EM structure of rnc-rac complex in presence of ssb from s. cerevisiae 2
- Reference
- Chen Y, Tsai B, Li N, Gao N (2022): "Structural remodeling of ribosome associated Hsp40-Hsp70 chaperones during co-translational folding." Nat Commun, 13, 3410. doi: 10.1038/s41467-022-31127-4.
- Abstract
- Ribosome associated complex (RAC), an obligate heterodimer of HSP40 and HSP70 (Zuo1 and Ssz1 in yeast), is conserved in eukaryotes and functions as co-chaperone for another HSP70 (Ssb1/2 in yeast) to facilitate co-translational folding of nascent polypeptides. Many mechanistic details, such as the coordination of one HSP40 with two HSP70s and the dynamic interplay between RAC-Ssb and growing nascent chains, remain unclear. Here, we report three sets of structures of RAC-containing ribosomal complexes isolated from Saccharomyces cerevisiae. Structural analyses indicate that RAC on the nascent-chain-free ribosome is in an autoinhibited conformation, and in the presence of a nascent chain at the peptide tunnel exit (PTE), RAC undergoes large-scale structural remodeling to make Zuo1 J-Domain more accessible to Ssb. Our data also suggest a role of Zuo1 in orienting Ssb-SBD proximal to the PTE for easy capture of the substrate. Altogether, in accordance with previous data, our work suggests a sequence of structural remodeling events for RAC-Ssb during co-translational folding, triggered by the binding and passage of growing nascent chain from one to another.