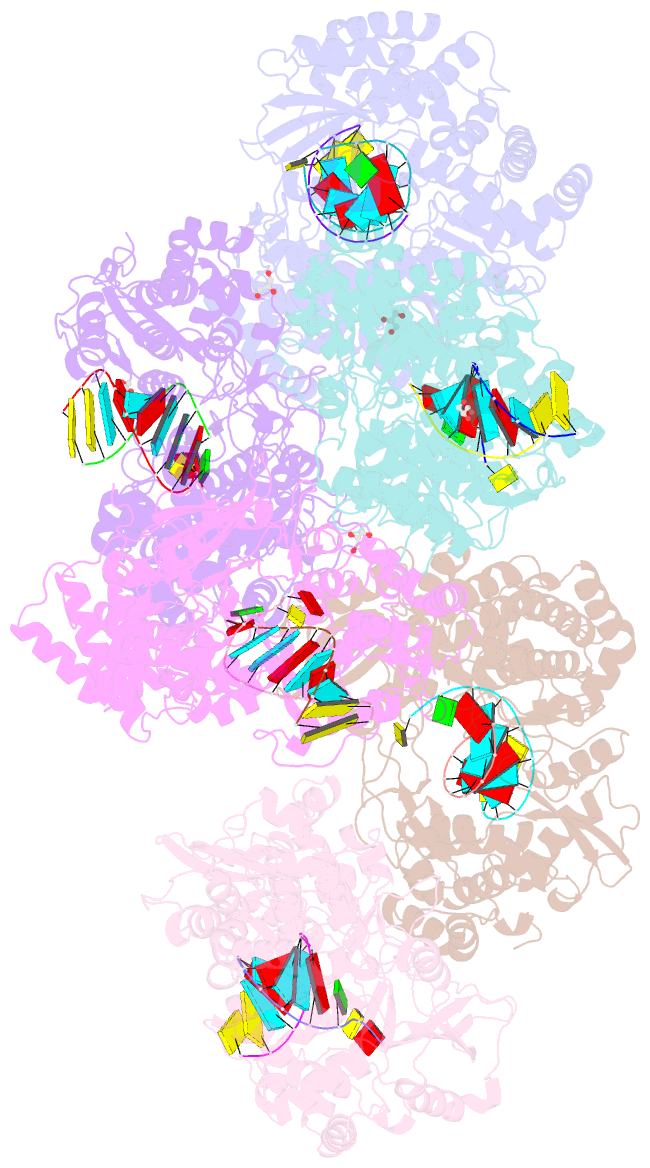
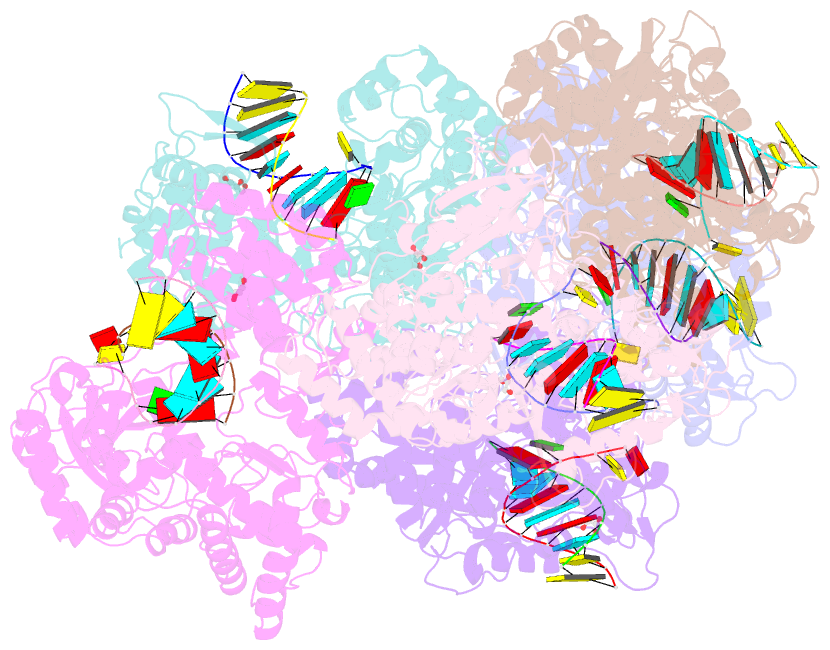
Summary information and primary citation
- PDB-id
- 7xd8; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-RNA
- Method
- X-ray (2.85 Å)
- Summary
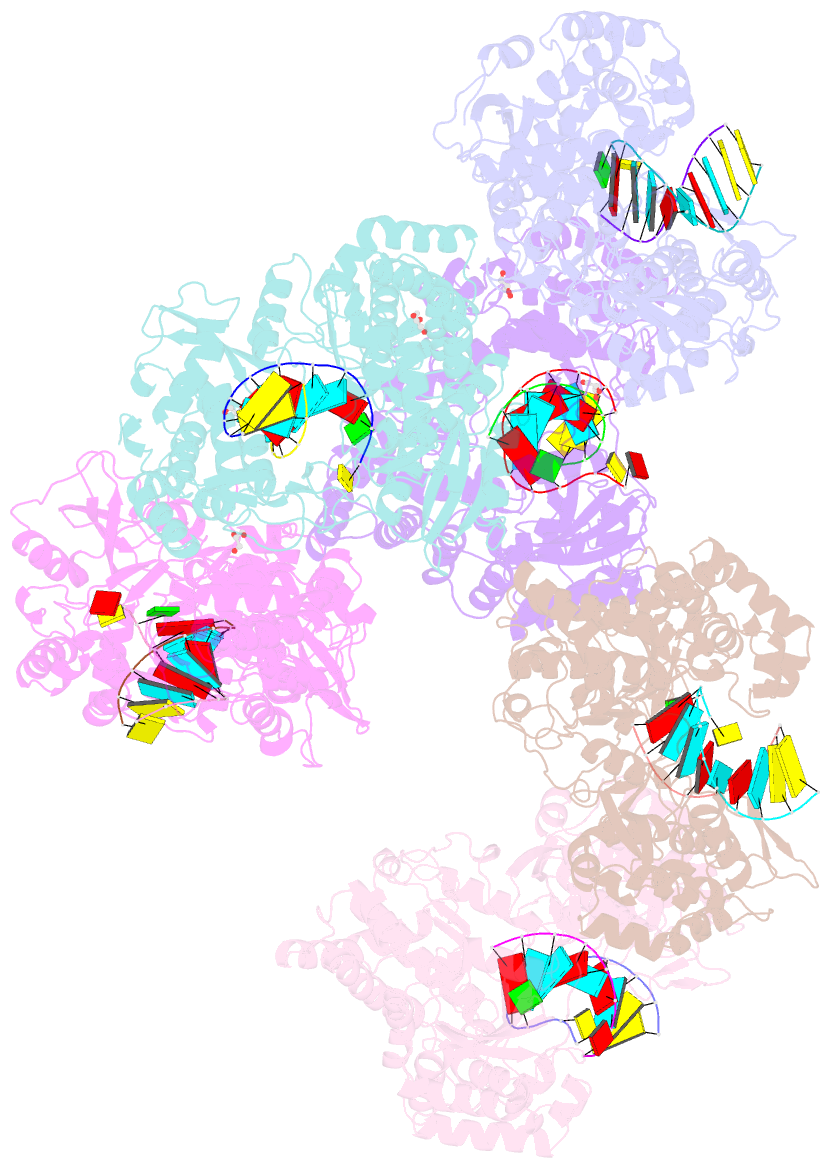
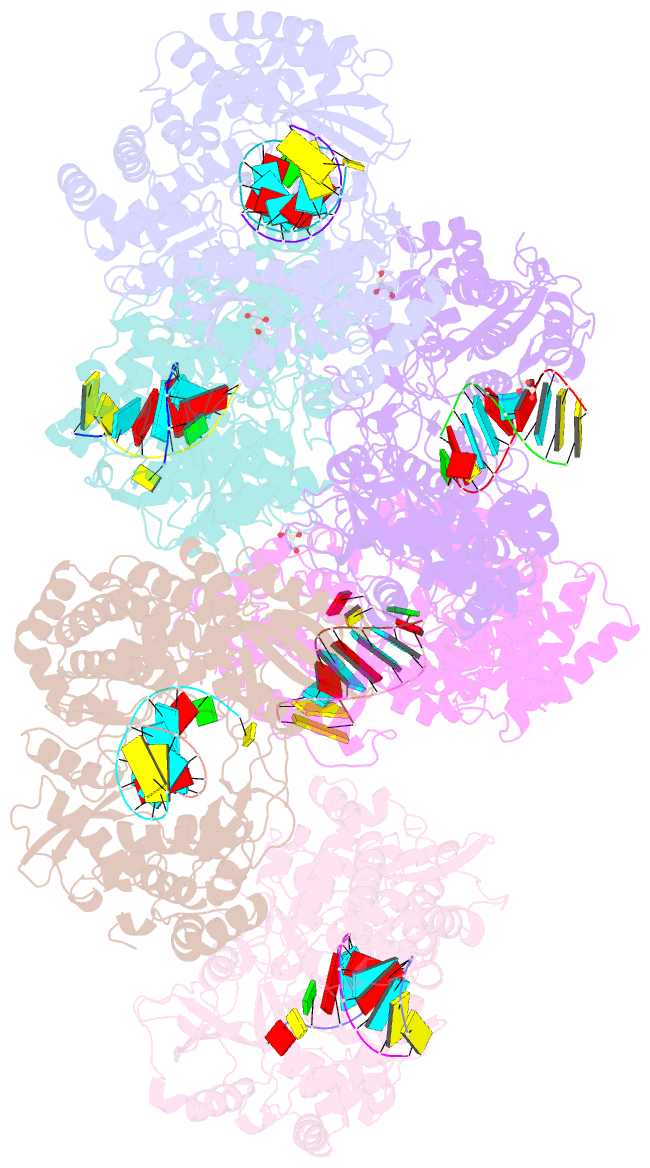
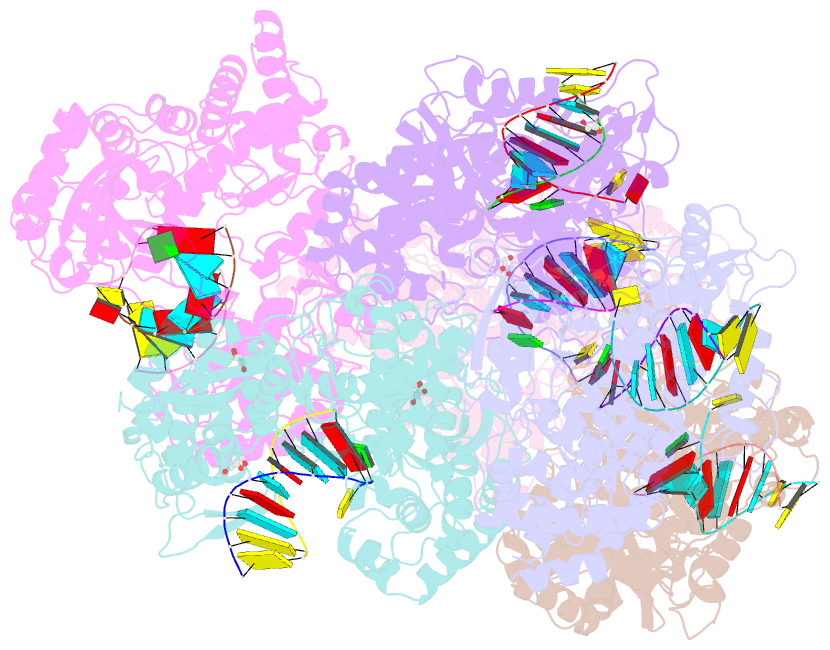
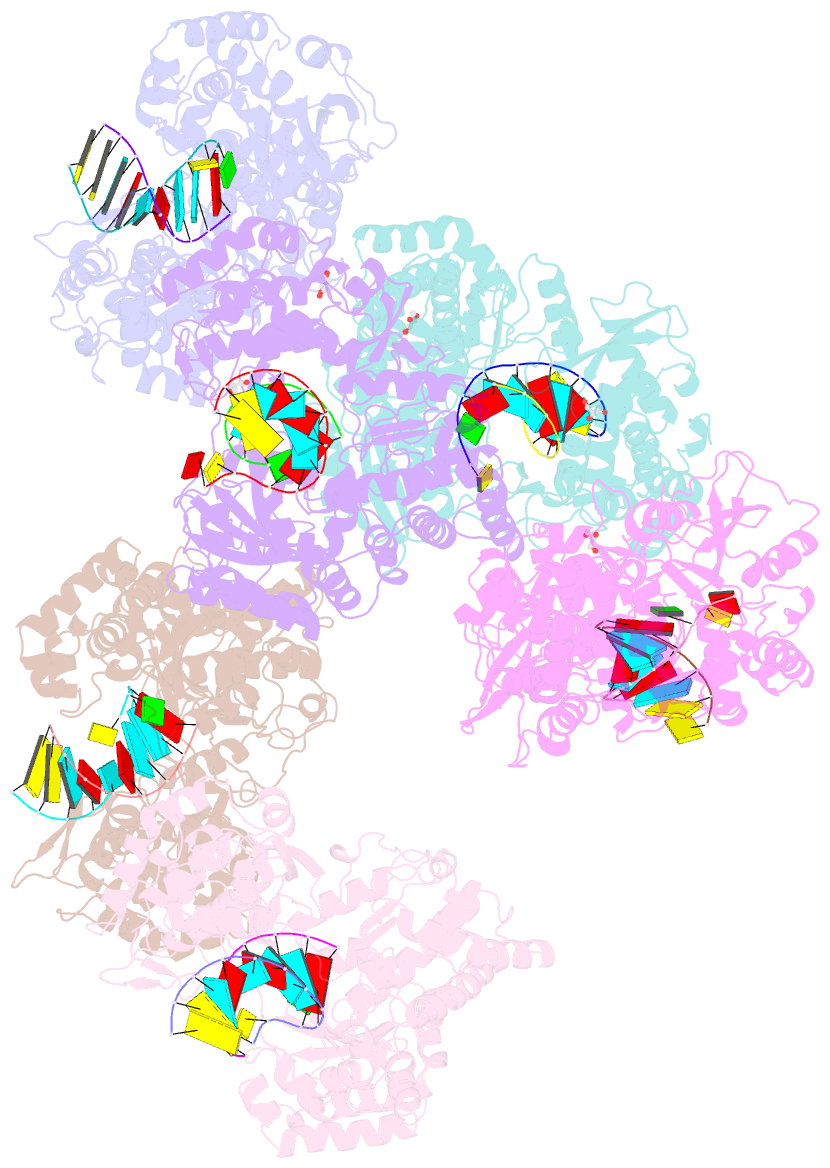
- Crystal structure of dengue virus serotype 2 (denv2) polymerase elongation complex (native form)
- Reference
- Wu J, Wang X, Liu Q, Lu G, Gong P (2023): "Structural basis of transition from initiation to elongation in de novo viral RNA-dependent RNA polymerases." Proc.Natl.Acad.Sci.USA, 120, e2211425120. doi: 10.1073/pnas.2211425120.
- Abstract
- De novo viral RNA-dependent RNA polymerases (RdRPs) utilize their priming element (PE) to facilitate accurate initiation. Upon transition to elongation, the PE has to retreat from the active site to give room to the template-product RNA duplex. However, PE conformational change upon this transition and the role of PE at elongation both remain elusive. Here, we report crystal structures of RdRP elongation complex (EC) from dengue virus serotype 2 (DENV2), demonstrating a dramatic refolding of PE that allows establishment of interactions with the RNA duplex backbone approved to be essential for EC stability. Enzymology data from both DENV2 and hepatitis C virus (HCV) RdRPs suggest that critical transition of the refolding likely occurs after synthesis of a 4- to 5-nucleotide (nt) product together providing a key basis in understanding viral RdRP transition from initiation to elongation.