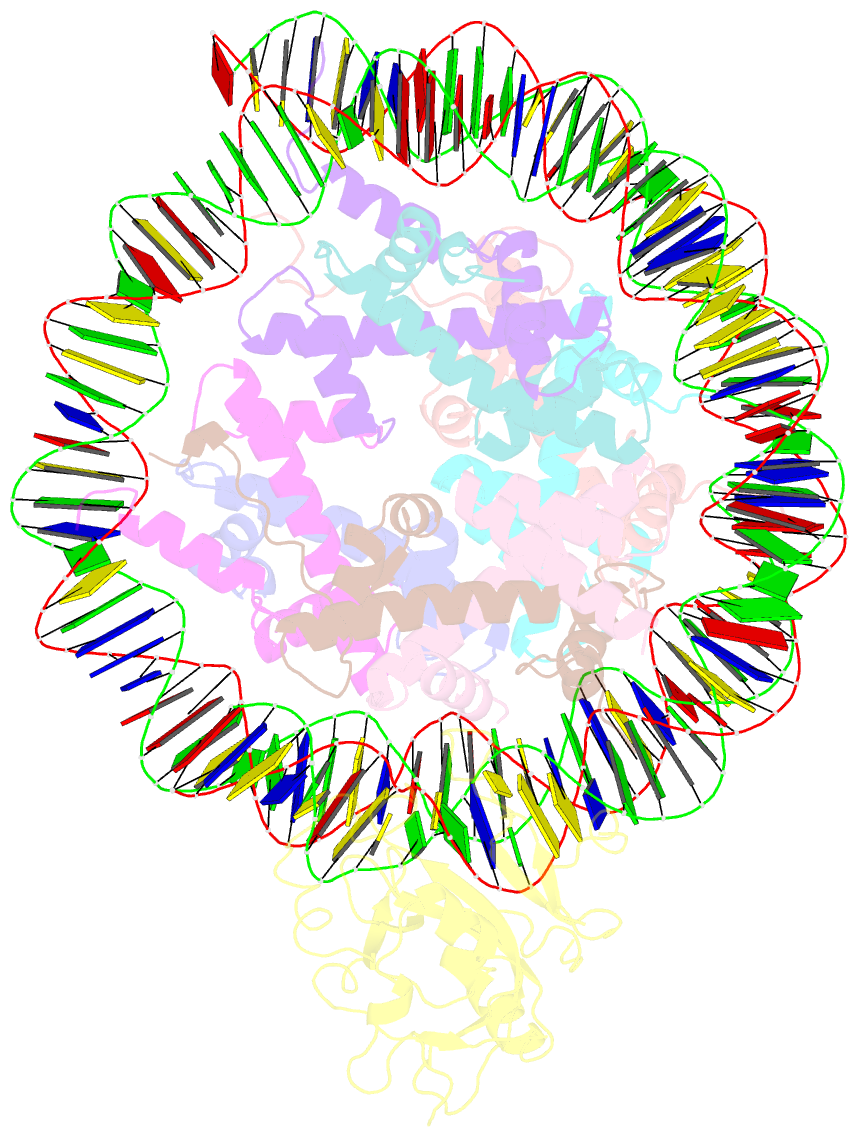
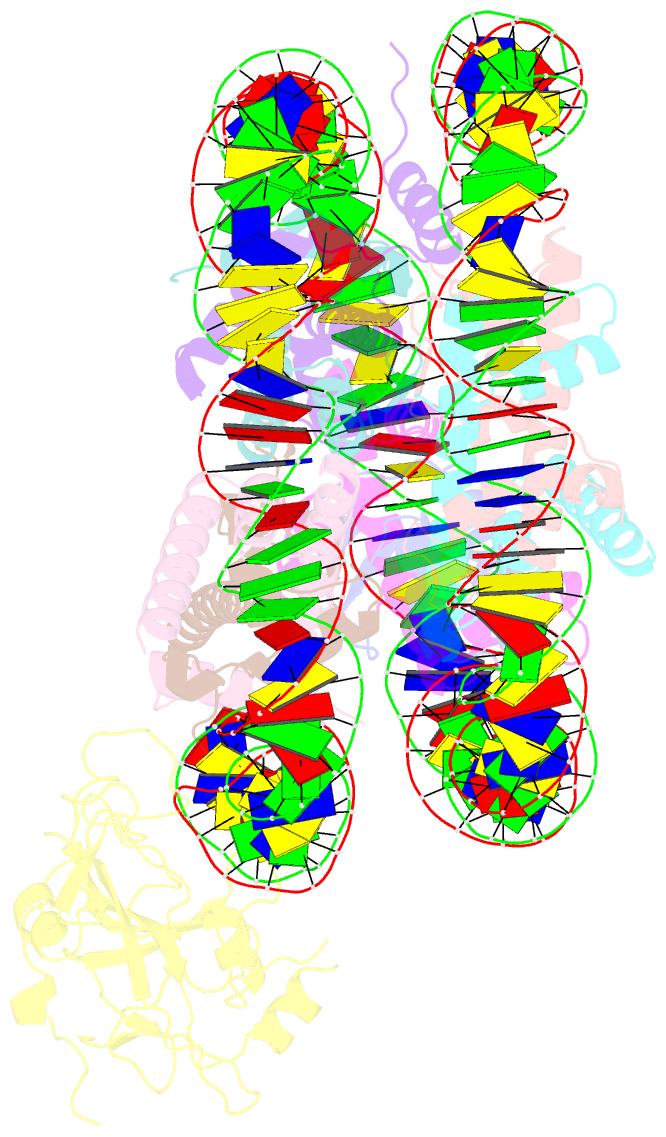
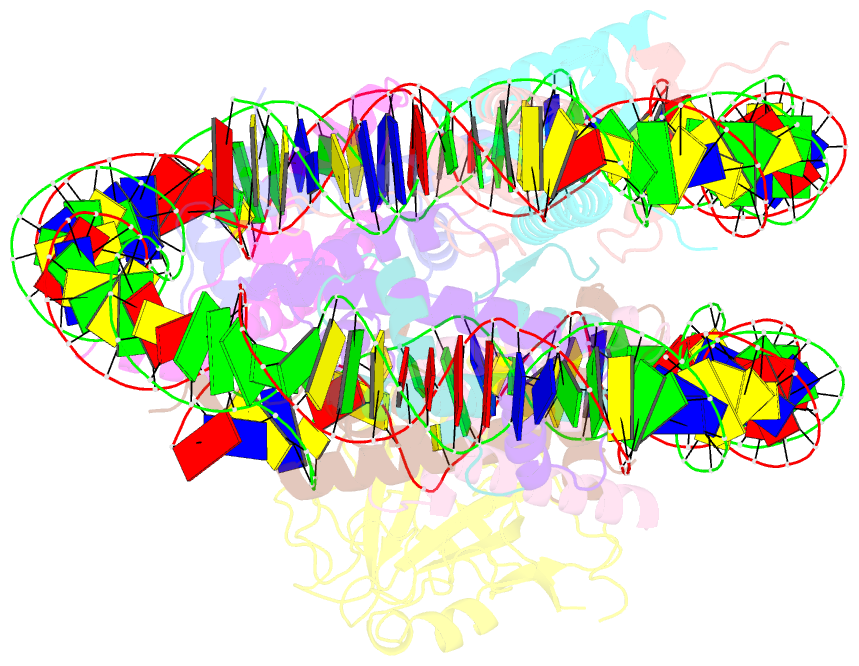
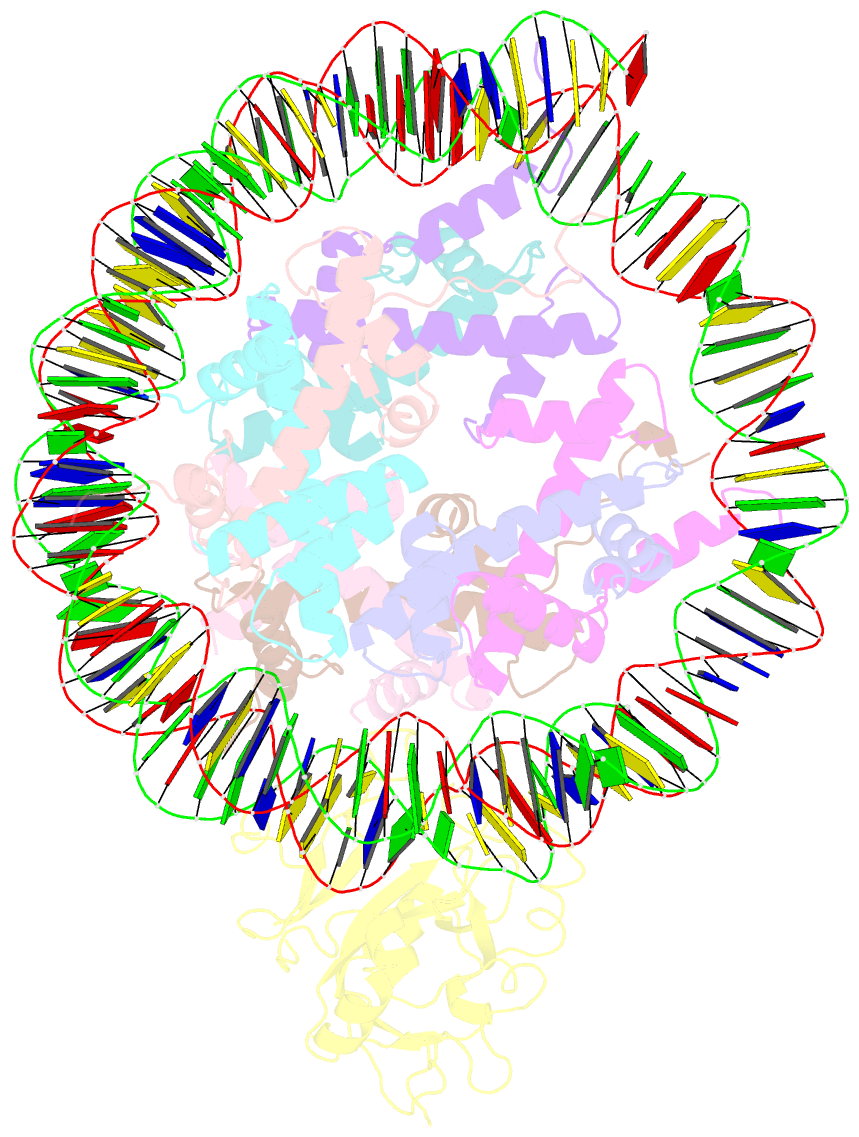
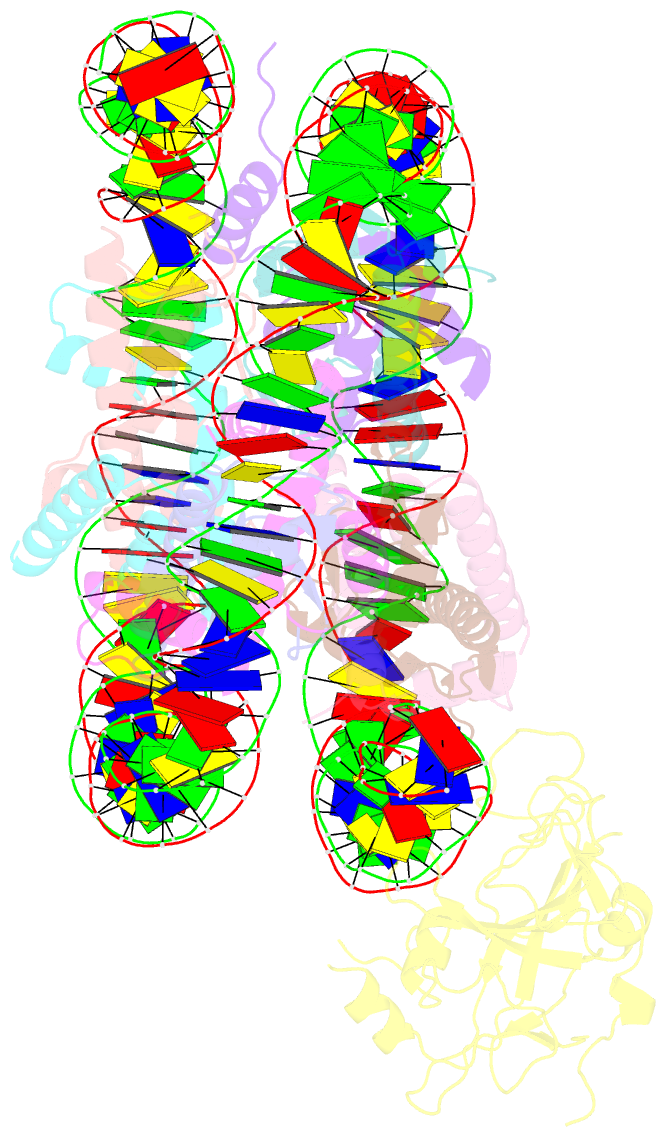
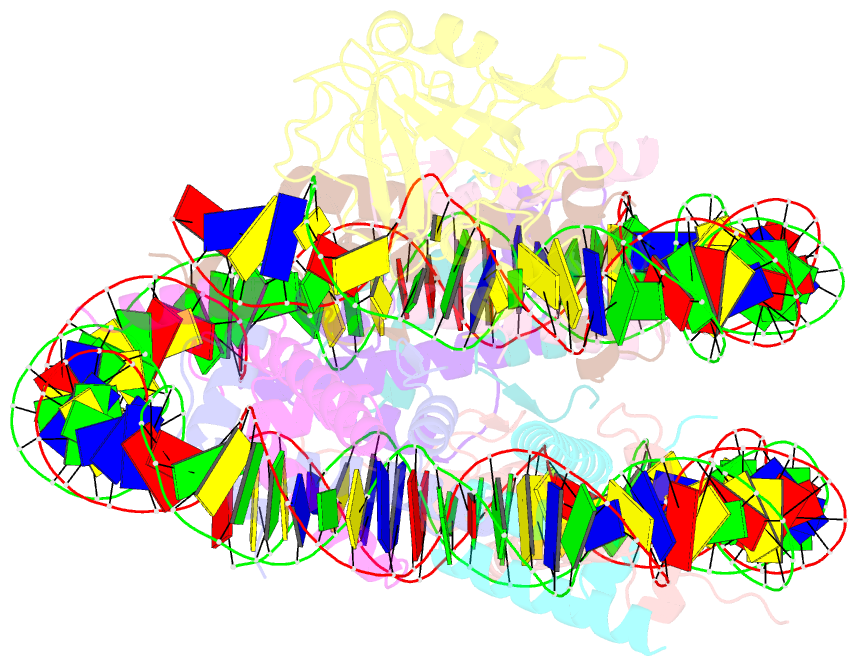
Summary information and primary citation
- PDB-id
- 7xfm; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- cryo-EM (3.1 Å)
- Summary
- Structure of nucleosome-aag complex (a-53i, post-catalytic state)
- Reference
- Zheng L, Tsai B, Gao N (2023): "Structural and mechanistic insights into the DNA glycosylase AAG-mediated base excision in nucleosome." Cell Discov, 9, 62. doi: 10.1038/s41421-023-00560-0.
- Abstract
- The engagement of a DNA glycosylase with a damaged DNA base marks the initiation of base excision repair. Nucleosome-based packaging of eukaryotic genome obstructs DNA accessibility, and how DNA glycosylases locate the substrate site on nucleosomes is currently unclear. Here, we report cryo-electron microscopy structures of nucleosomes bearing a deoxyinosine (DI) in various geometric positions and structures of them in complex with the DNA glycosylase AAG. The apo nucleosome structures show that the presence of a DI alone perturbs nucleosomal DNA globally, leading to a general weakening of the interface between DNA and the histone core and greater flexibility for the exit/entry of the nucleosomal DNA. AAG makes use of this nucleosomal plasticity and imposes further local deformation of the DNA through formation of the stable enzyme-substrate complex. Mechanistically, local distortion augmentation, translation/rotational register shift and partial opening of the nucleosome are employed by AAG to cope with substrate sites in fully exposed, occluded and completely buried positions, respectively. Our findings reveal the molecular basis for the DI-induced modification on the structural dynamics of the nucleosome and elucidate how the DNA glycosylase AAG accesses damaged sites on the nucleosome with different solution accessibility.