Summary information and primary citation
- PDB-id
- 7xjg; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-DNA-RNA
- Method
- cryo-EM (2.51 Å)
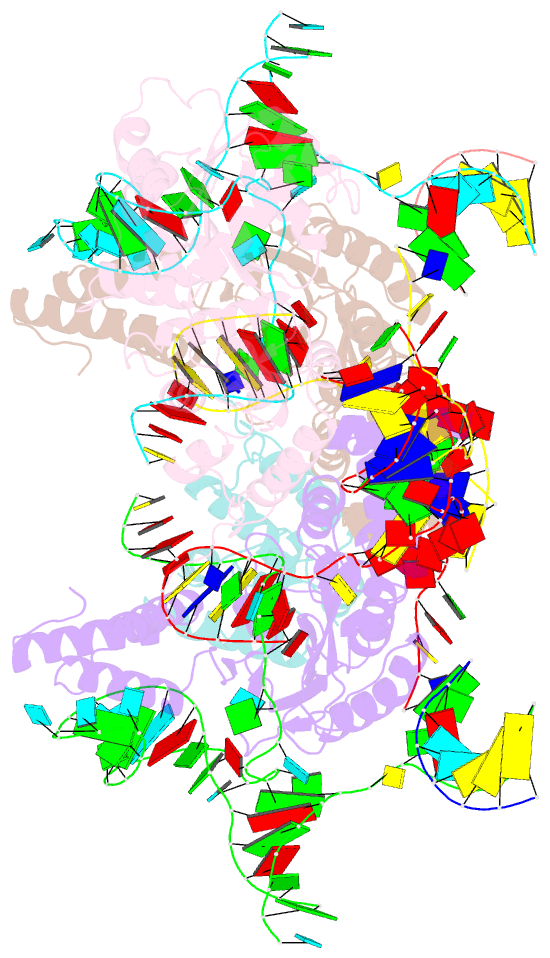
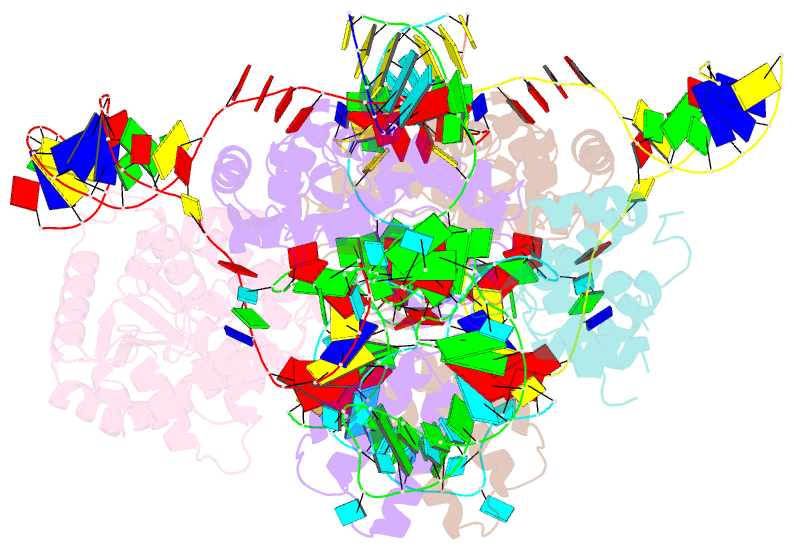
- Summary
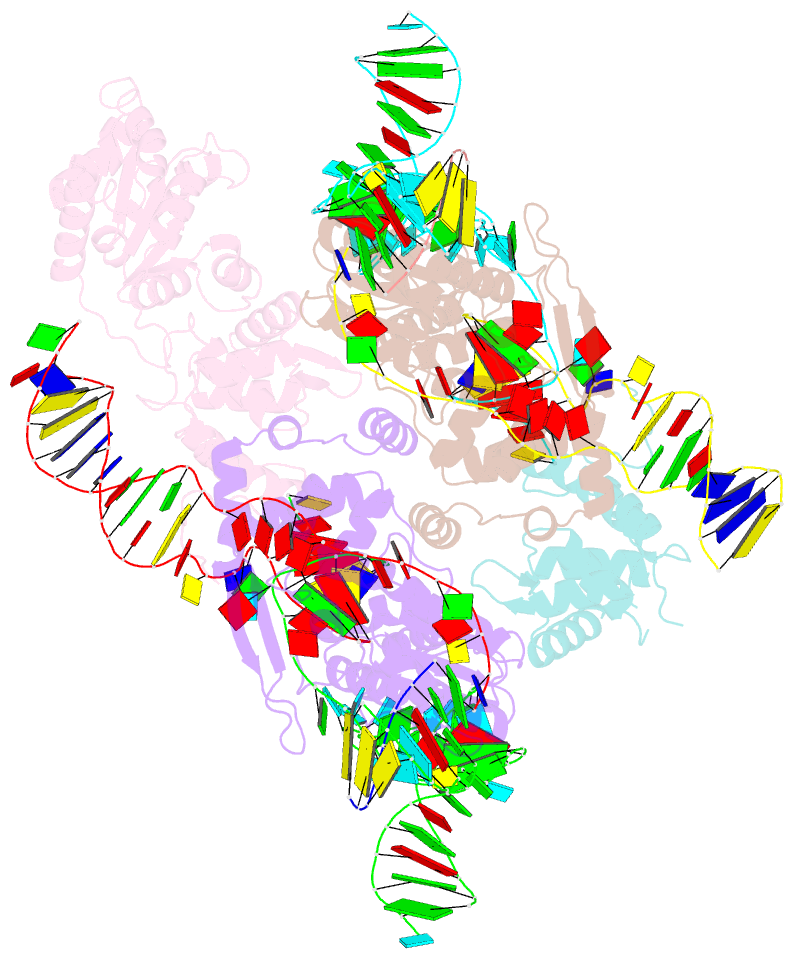
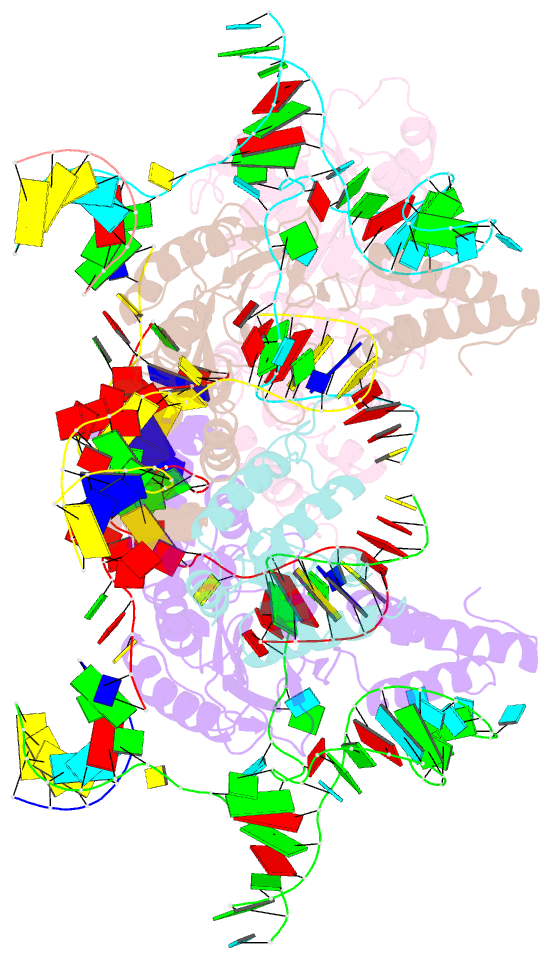
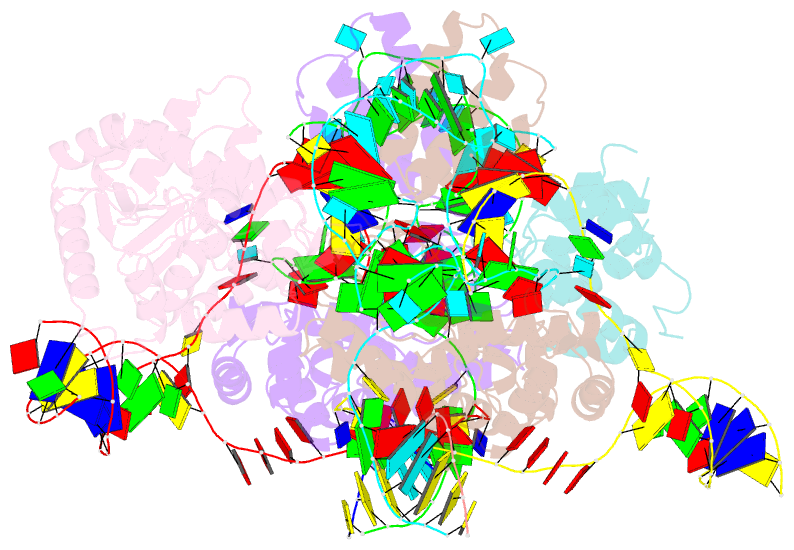
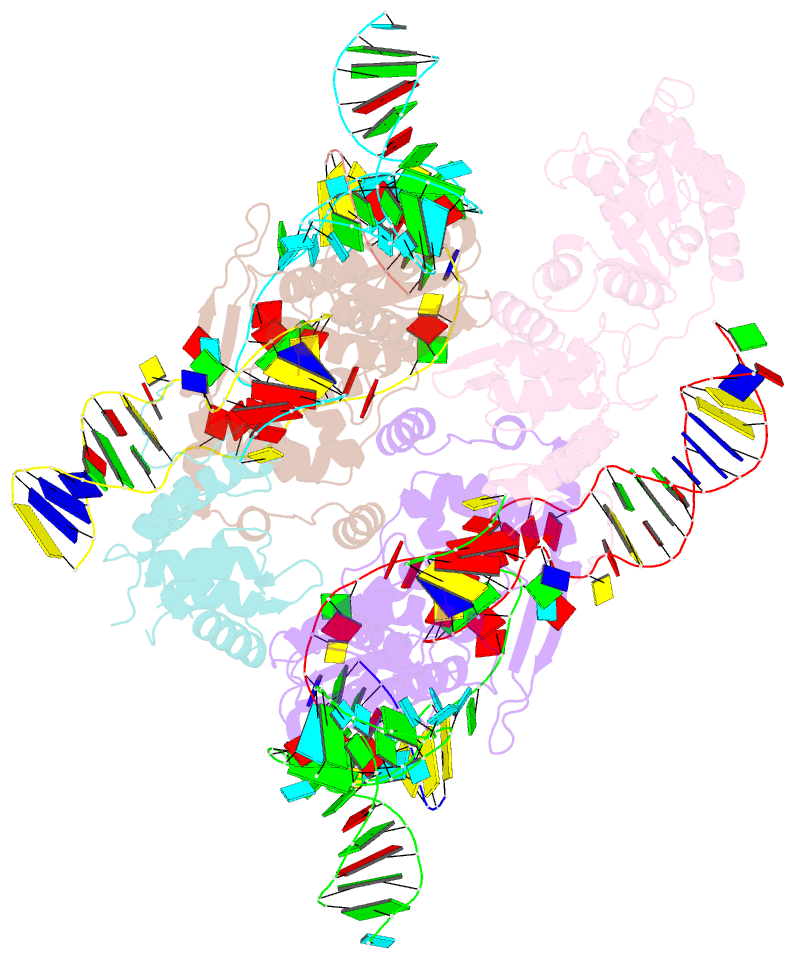
- cryo-EM structure of e.coli retron-ec86 in complex with its effector at 2.5 angstrom
- Reference
- Wang Y, Guan Z, Wang C, Nie Y, Chen Y, Qian Z, Cui Y, Xu H, Wang Q, Zhao F, Zhang D, Tao P, Sun M, Yin P, Jin S, Wu S, Zou T (2022): "Cryo-EM structures of Escherichia coli Ec86 retron complexes reveal architecture and defence mechanism." Nat Microbiol, 7, 1480-1489. doi: 10.1038/s41564-022-01197-7.
- Abstract
- First discovered in the 1980s, retrons are bacterial genetic elements consisting of a reverse transcriptase and a non-coding RNA (ncRNA). Retrons mediate antiphage defence in bacteria but their structure and defence mechanisms are unknown. Here, we investigate the Escherichia coli Ec86 retron and use cryo-electron microscopy to determine the structures of the Ec86 (3.1 Å) and cognate effector-bound Ec86 (2.5 Å) complexes. The Ec86 reverse transcriptase exhibits a characteristic right-hand-like fold consisting of finger, palm and thumb subdomains. Ec86 reverse transcriptase reverse-transcribes part of the ncRNA into satellite, multicopy single-stranded DNA (msDNA, a DNA-RNA hybrid) that we show wraps around the reverse transcriptase electropositive surface. In msDNA, both inverted repeats are present and the 3' sides of the DNA/RNA chains are close to the reverse transcriptase active site. The Ec86 effector adopts a two-lobe fold and directly binds reverse transcriptase and msDNA. These findings offer insights into the structure-function relationship of the retron-effector unit and provide a structural basis for the optimization of retron-based genome editing systems.