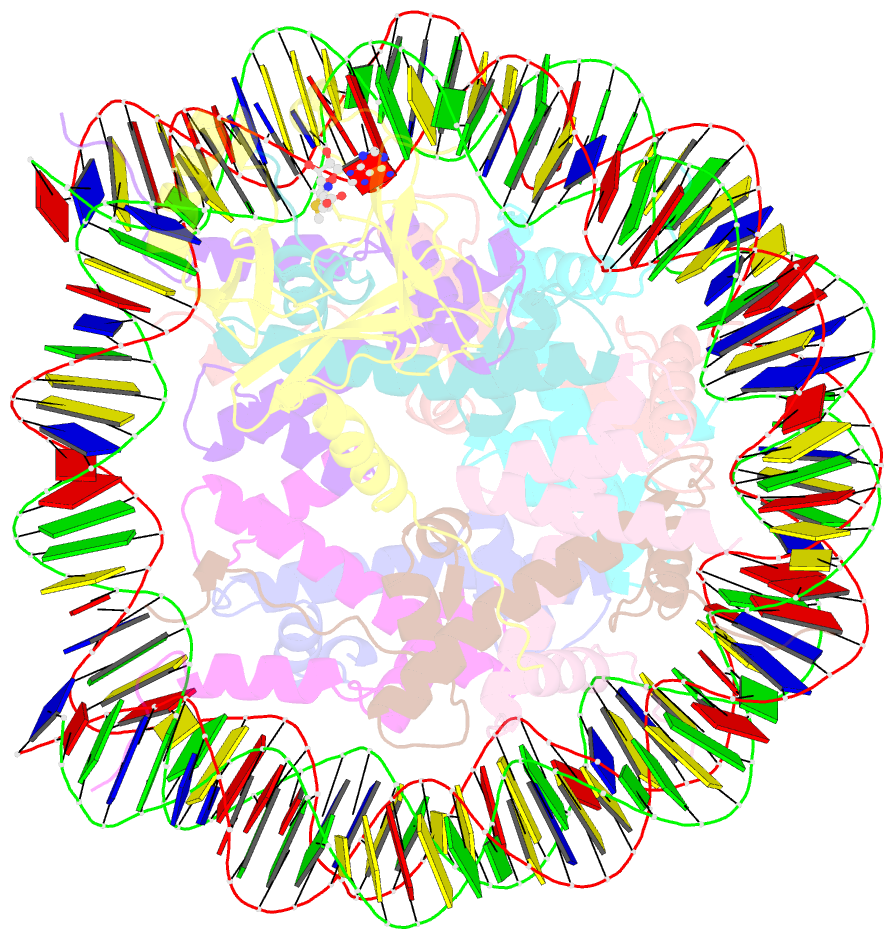
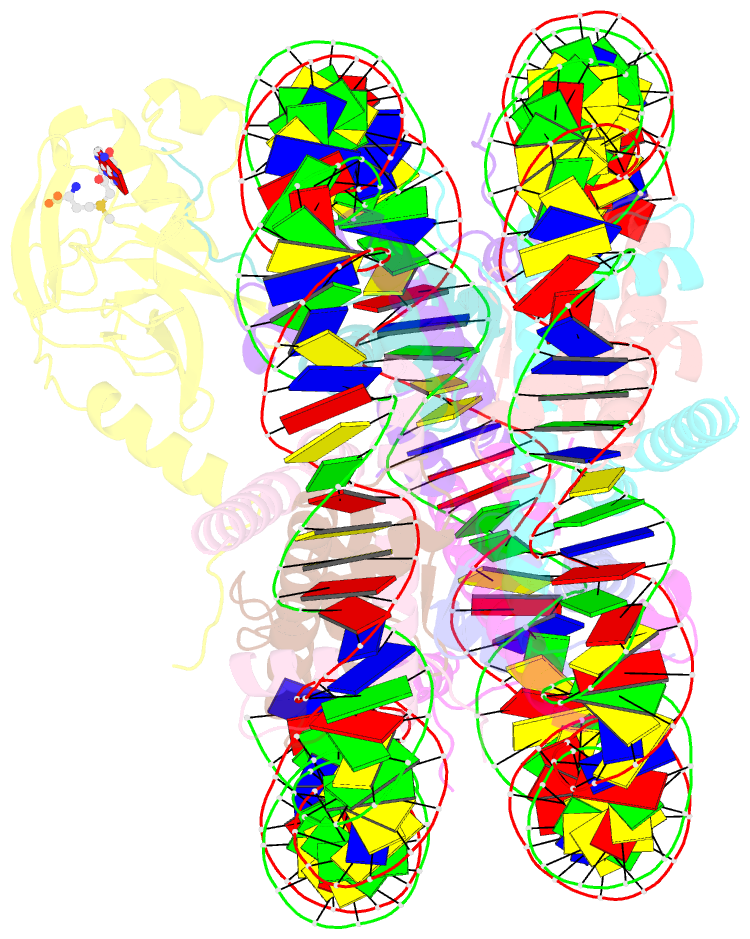
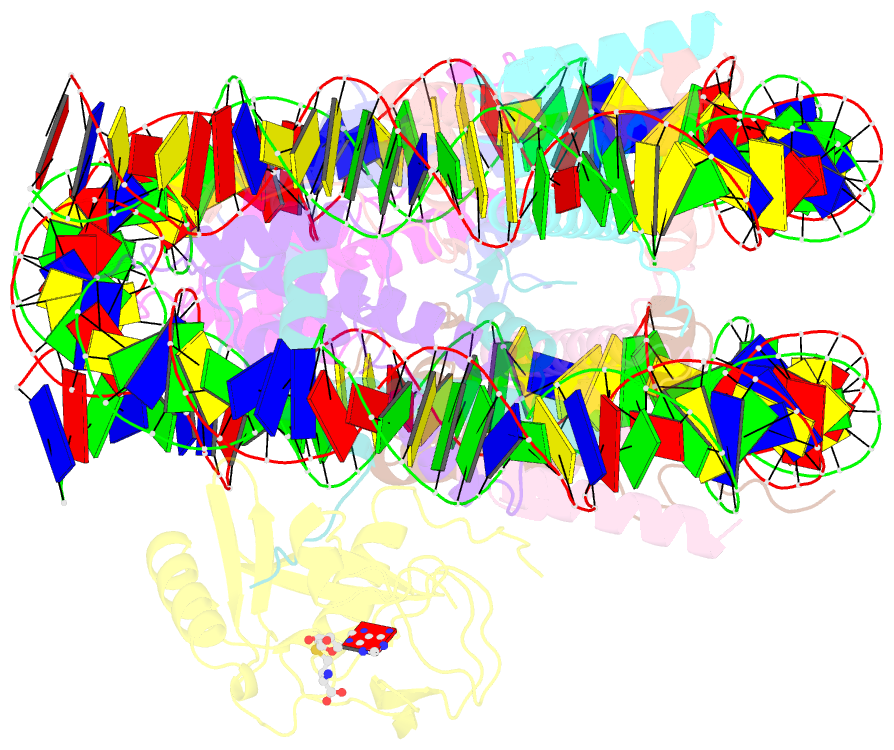
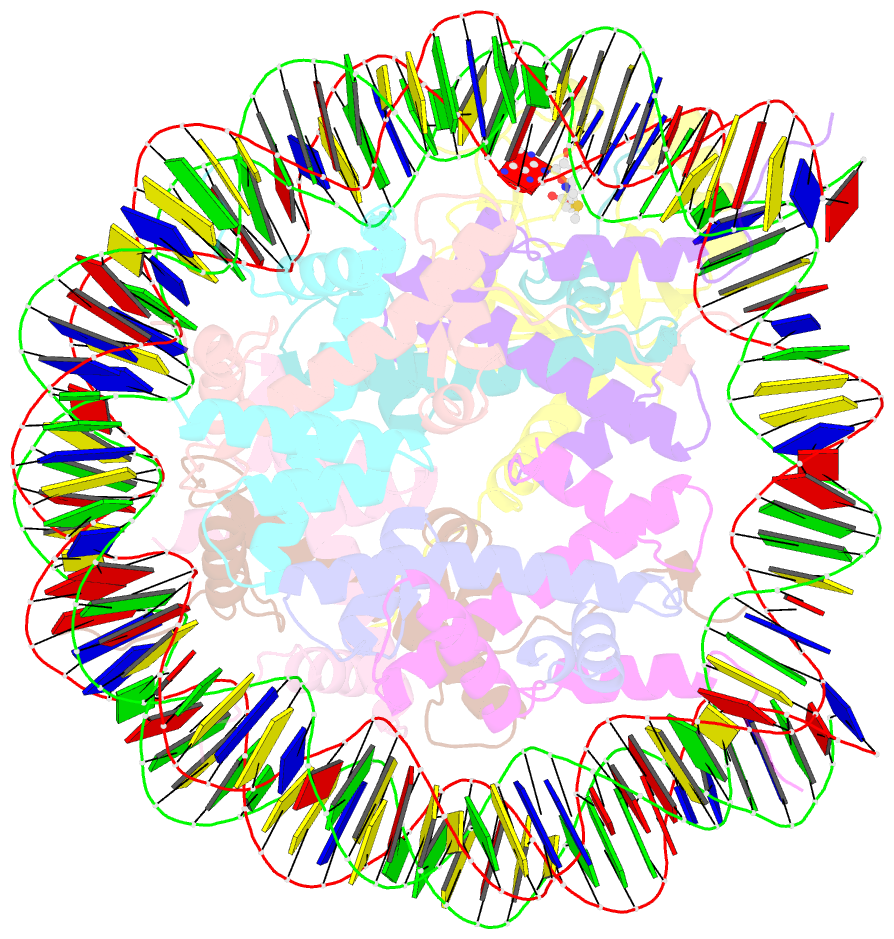
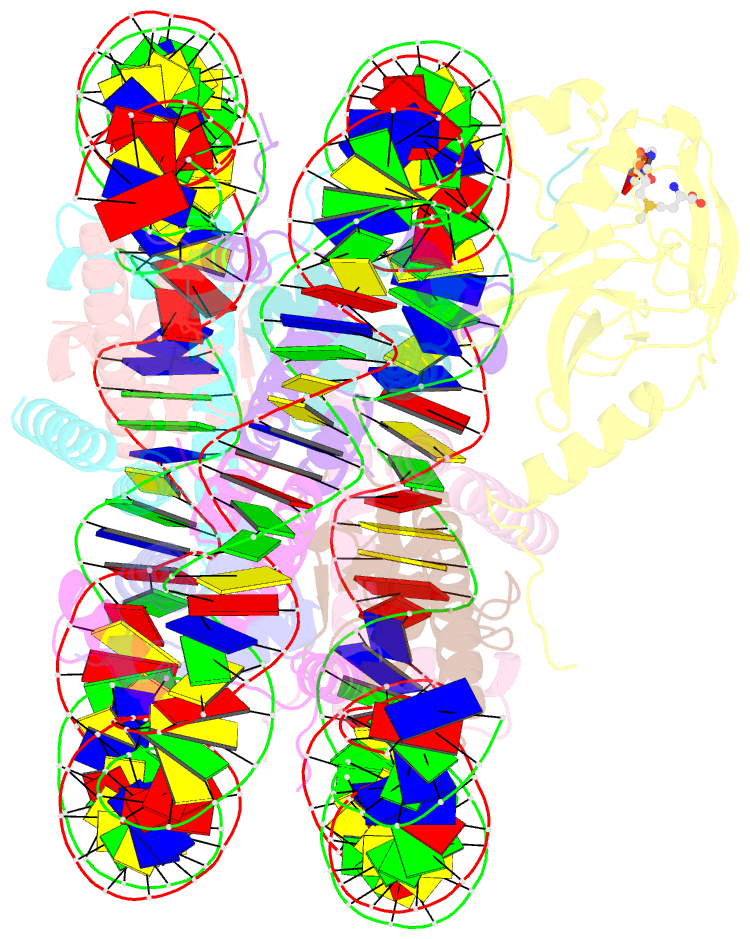
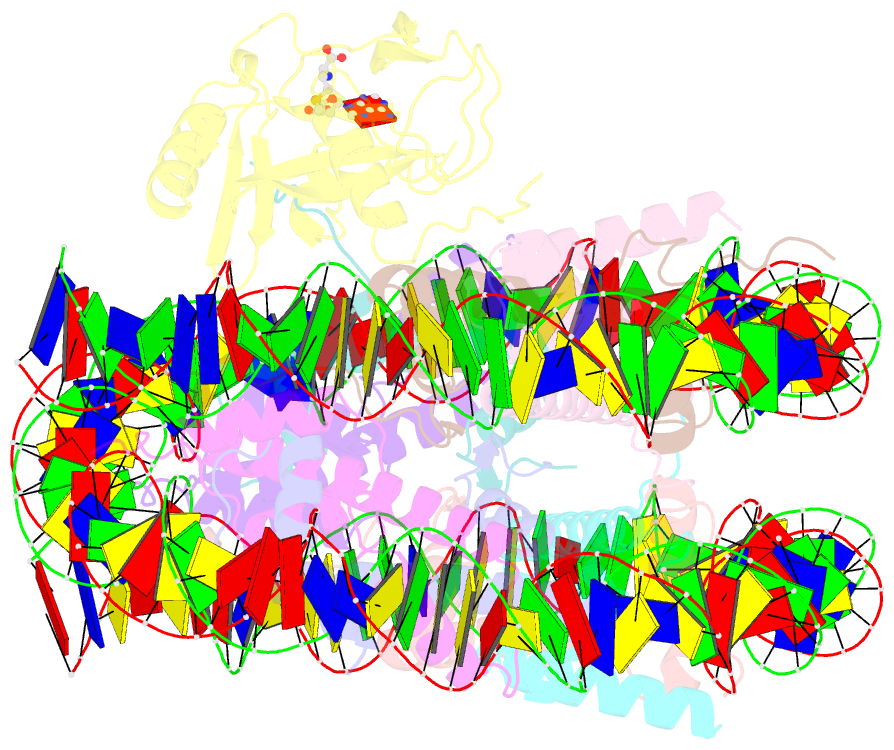
Summary information and primary citation
- PDB-id
- 7xpx; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- nuclear protein-DNA
- Method
- cryo-EM (3.2 Å)
- Summary
- cryo-EM structure of the histone methyltransferase set8 bound to h4k20ecx-nucleosome
- Reference
- Shi L, Huang L, Long H, Song A, Zhou Z (2022): "Structural basis of nucleosomal H4K20 methylation by methyltransferase SET8." Faseb J., 36, e22338. doi: 10.1096/fj.202101821R.
- Abstract
- Histone H4 lysine 20 monomethylation (H4K20me1) plays a crucial role in multiple processes including DNA damage repair, DNA replication, and cell cycle control. Histone methyltransferase SET8 (previously named PR-Set7/KMT5A) mediates the chromatin deposition of H4K20me1, but how SET8 recognizes and modifies H4 in the context of the nucleosome is not fully understood. Here, we developed a simple chemical modification approach for H4K20 substitution by using the lysine analog S-ethyl-L-cysteine (Ecx). Substitution of H4K20 with H4Ecx20 improves the stability of the SET8-nucleosome complex, allowing us to determine the cryo-EM structure at 3.2 Å resolution. Structural analyses show that SET8 directly interacts with the H4 tail and the H2A-H2B acidic patch to ensure nucleosome binding. SET8 residues R339, K341, K351 make contact with nucleosomal DNA at the super helical location 2 (SHL2). Substitution of SET8 DNA-binding residues with alanines decreases the SET8-nucleosome interaction and impairs the methyltransferase activity. Disrupting the binding between SET8 R192 and H2A-H2B acidic patch decreases the cellular level of H4K20me1. Together, these results reveal a near-atomic resolution structure of SET8-bound nucleosome and provide insights into the SET8-mediated H4K20 recognition and modification. The lysine-to-Ecx substitution approach can be applied to the study of other methyltransferases.