Summary information and primary citation
- PDB-id
- 7xsq; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- immune system-RNA
- Method
- cryo-EM (2.88 Å)
- Summary
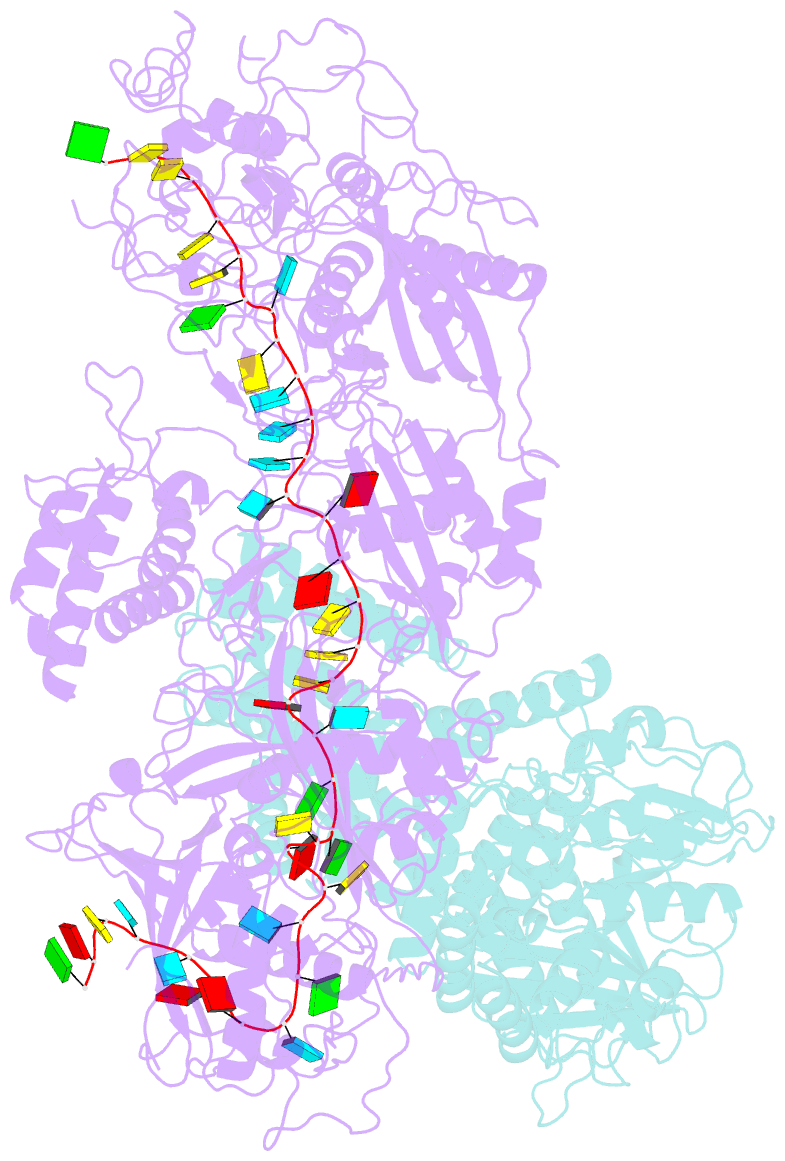
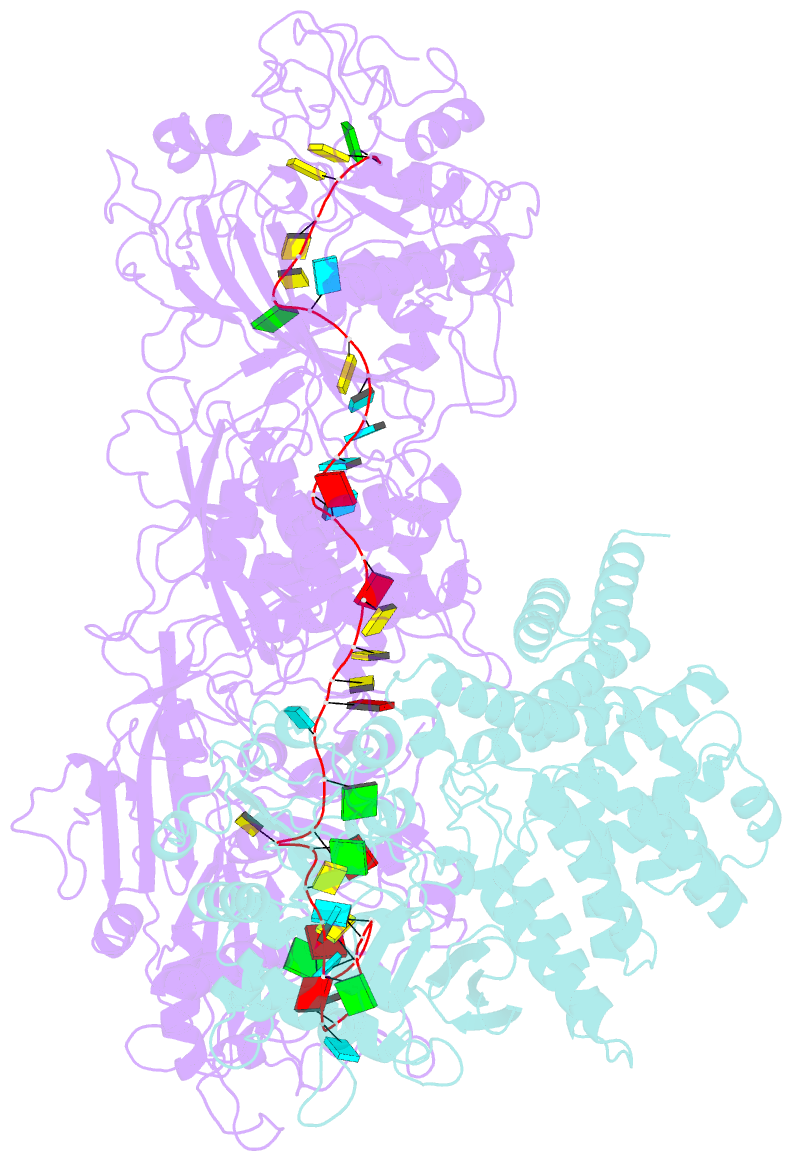
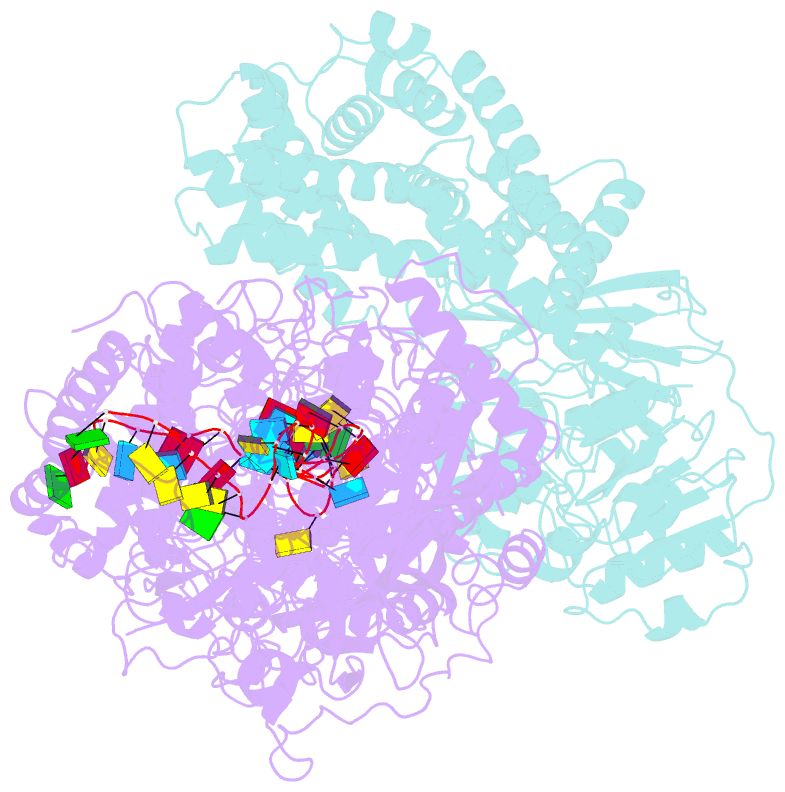
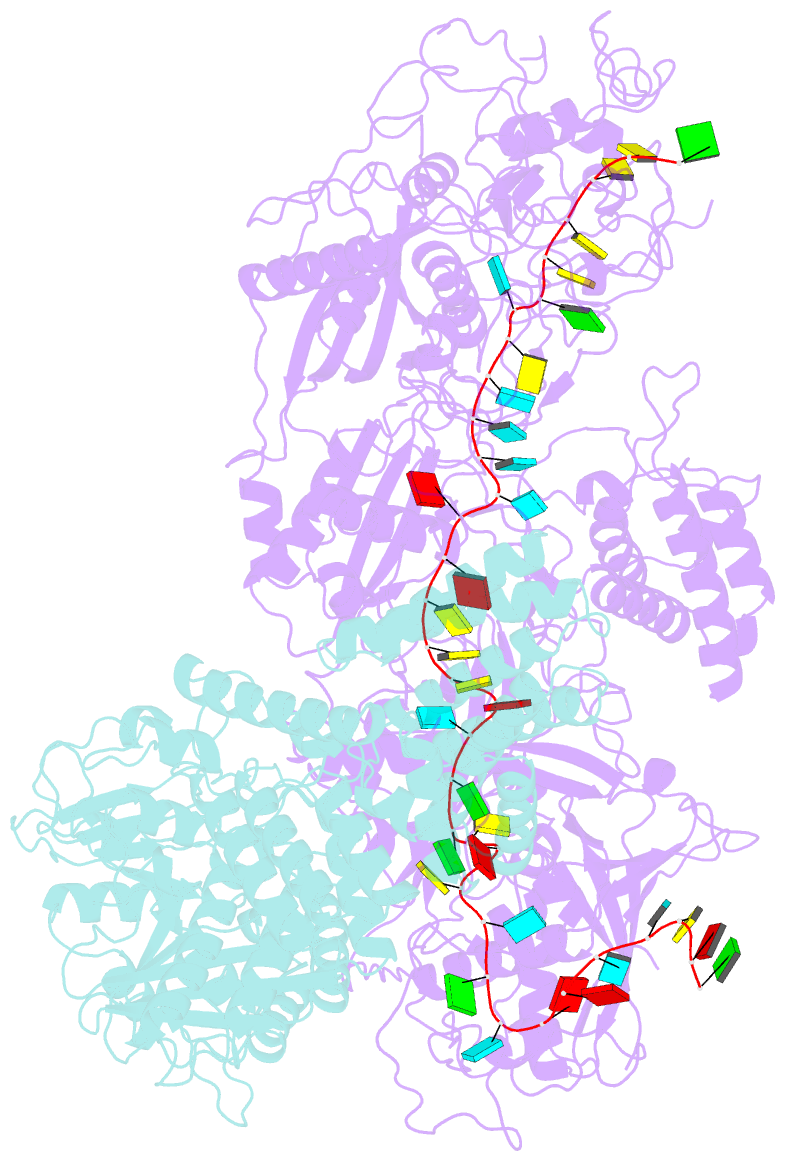
- Structure of the craspase
- Reference
- Liu X, Zhang L, Wang H, Xiu Y, Huang L, Gao Z, Li N, Li F, Xiong W, Gao T, Zhang Y, Yang M, Feng Y (2022): "Target RNA activates the protease activity of Craspase to confer antiviral defense." Mol.Cell, 82, 4503-4518.e8. doi: 10.1016/j.molcel.2022.10.007.
- Abstract
- In the type III-E CRISPR-Cas system, a Cas effector (gRAMP) is associated with a TPR-CHAT to form Craspase (CRISPR-guided caspase). However, both the structural features of gRAMP and the immunity mechanism remain unknown for this system. Here, we report structures of gRAMP-crRNA and gRAMP:cRNA:target RNA as well as structures of Craspase and Craspase complexed with cognate target RNA (CTR) or non-cognate target RNA (NTR). Importantly, the 3' anti-tag region of NTR and CTR binds at two distinct channels in Craspase, and CTR with a non-complementary 3' anti-tag induces a marked conformational change of the TPR-CHAT, which allosterically activates its protease activity to cleave an ancillary protein Csx30. This cleavage then triggers an abortive infection as the antiviral strategy of the type III-E system. Together, our study provides crucial insights into both the catalytic mechanism of the gRAMP and the immunity mechanism of the type III-E system.