Summary information and primary citation
- PDB-id
- 7yg7; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- virus like particle-RNA
- Method
- cryo-EM (3.7 Å)
- Summary
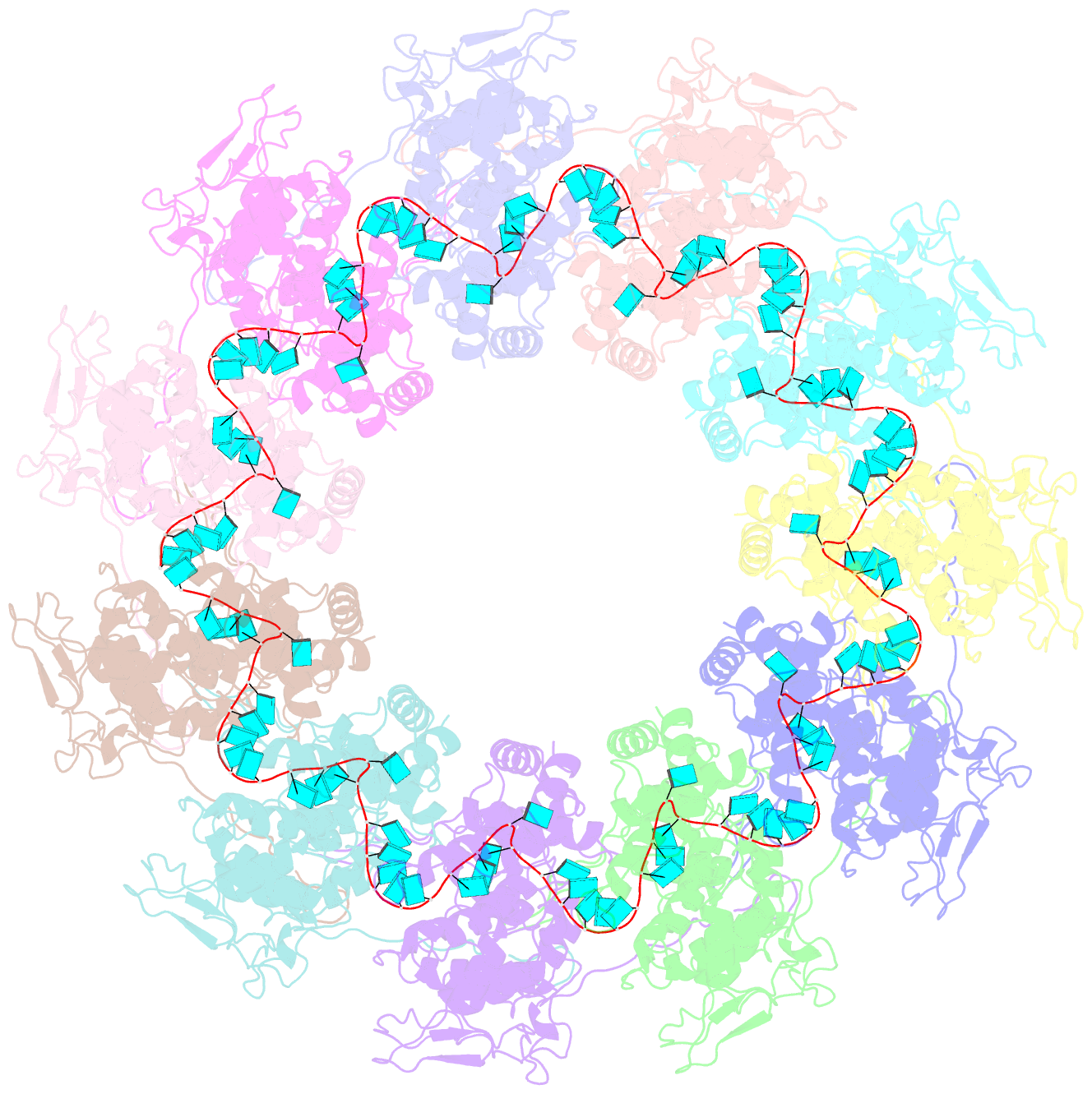
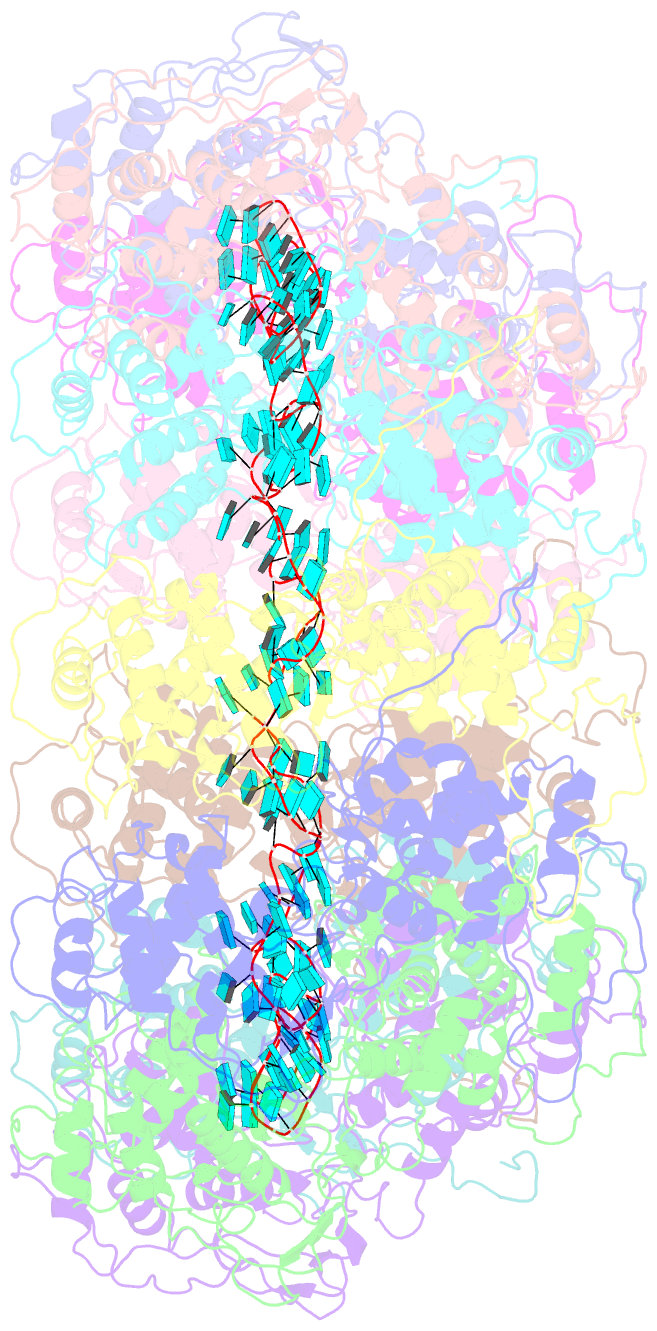
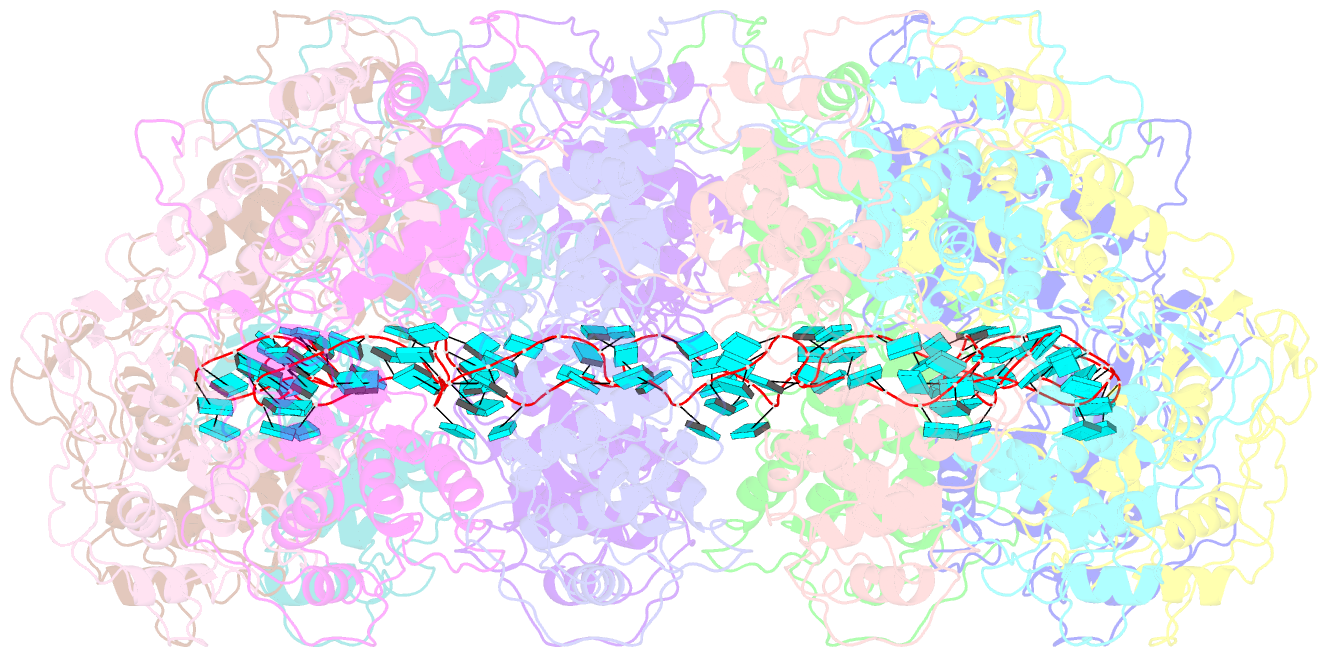
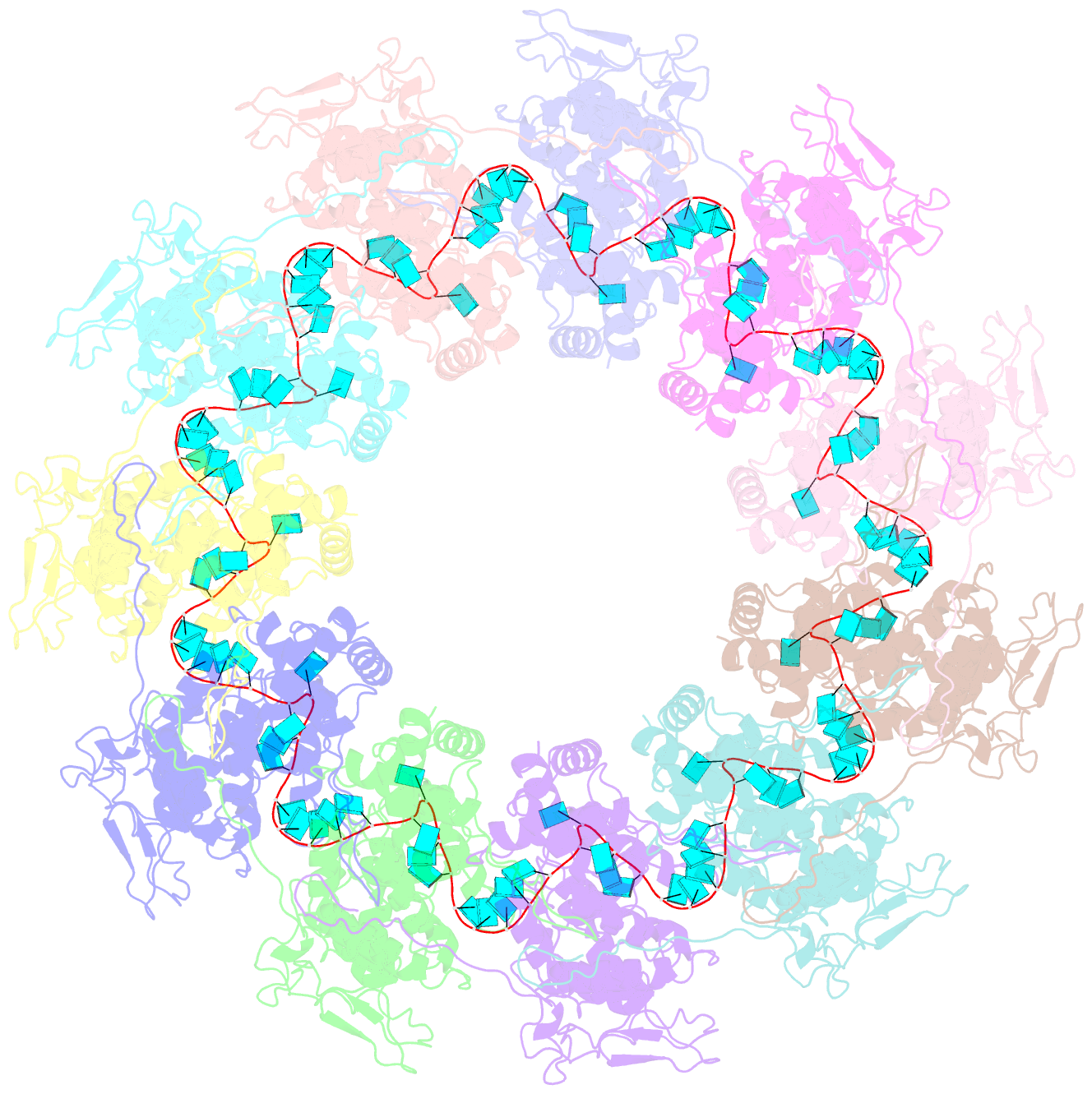
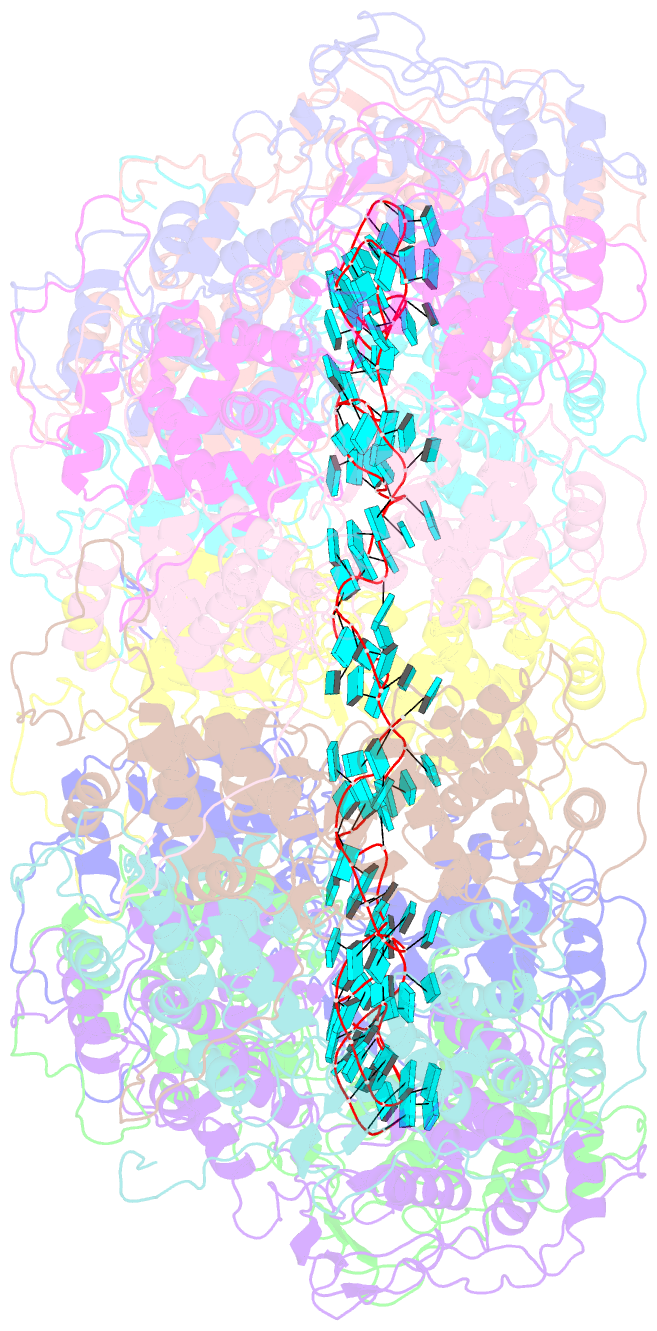
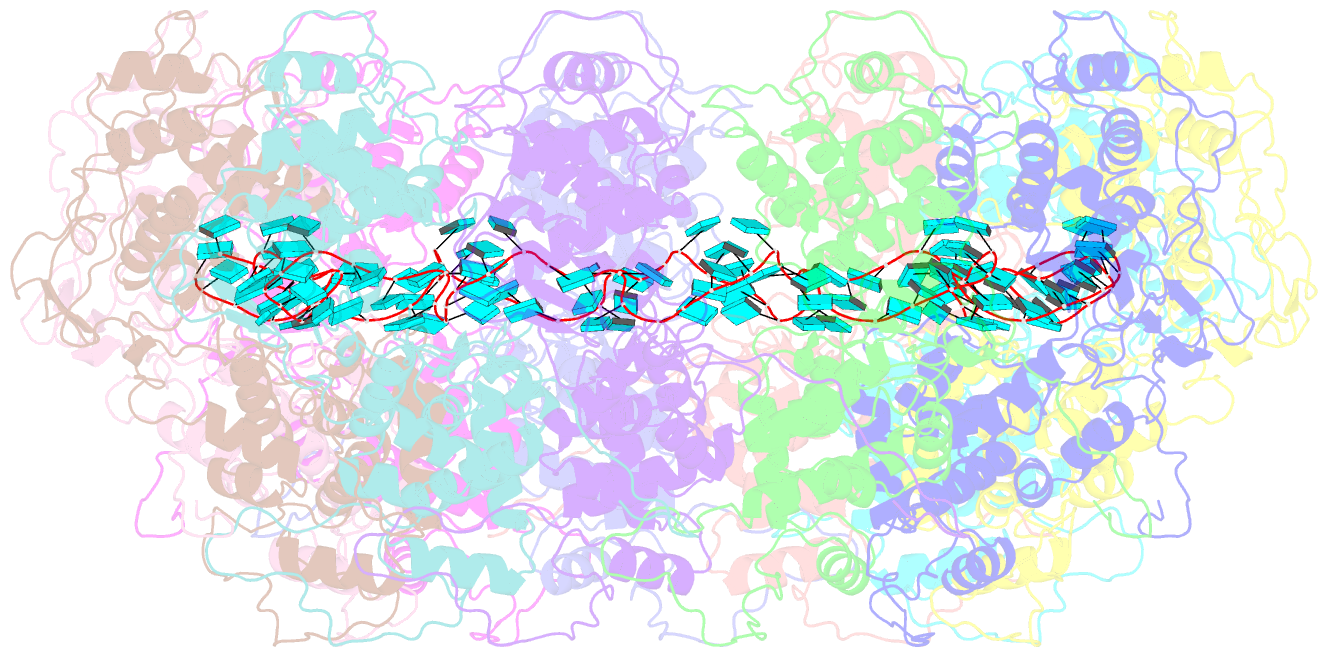
- Structure of the spring viraemia of carp virus ribonucleoprotein complex
- Reference
- Wang ZX, Liu B, Yang T, Yu D, Zhang C, Zheng L, Xie J, Liu B, Liu M, Peng H, Lai L, Ouyang Q, Ouyang S, Zhang YA (2023): "Structure of the Spring Viraemia of Carp Virus Ribonucleoprotein Complex Reveals Its Assembly Mechanism and Application in Antiviral Drug Screening." J.Virol., 97, e0182922. doi: 10.1128/jvi.01829-22.
- Abstract
- Spring viremia of carp virus (SVCV) is a highly pathogenic Vesiculovirus infecting the common carp, yet neither a vaccine nor effective therapies are available to treat spring viremia of carp (SVC). Like all negative-sense viruses, SVCV contains an RNA genome that is encapsidated by the nucleoprotein (N) in the form of a ribonucleoprotein (RNP) complex, which serves as the template for viral replication and transcription. Here, the three-dimensional (3D) structure of SVCV RNP was resolved through cryo-electron microscopy (cryo-EM) at a resolution of 3.7 Å. RNP assembly was stabilized by N and C loops; RNA was wrapped in the groove between the N and C lobes with 9 nt nucleotide per protomer. Combined with mutational analysis, our results elucidated the mechanism of RNP formation. The RNA binding groove of SVCV N was used as a target for drug virtual screening, and it was found suramin had a good antiviral effect. This study provided insights into RNP assembly, and anti-SVCV drug screening was performed on the basis of this structure, providing a theoretical basis and efficient drug screening method for the prevention and treatment of SVC.