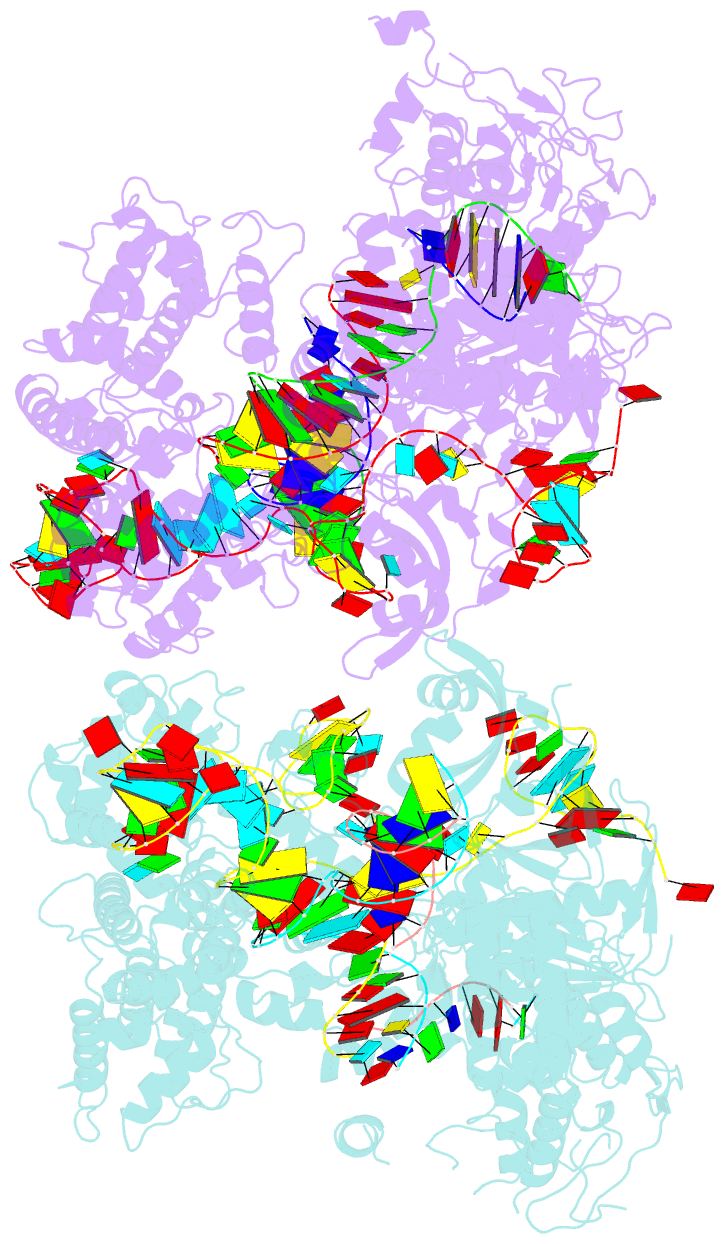
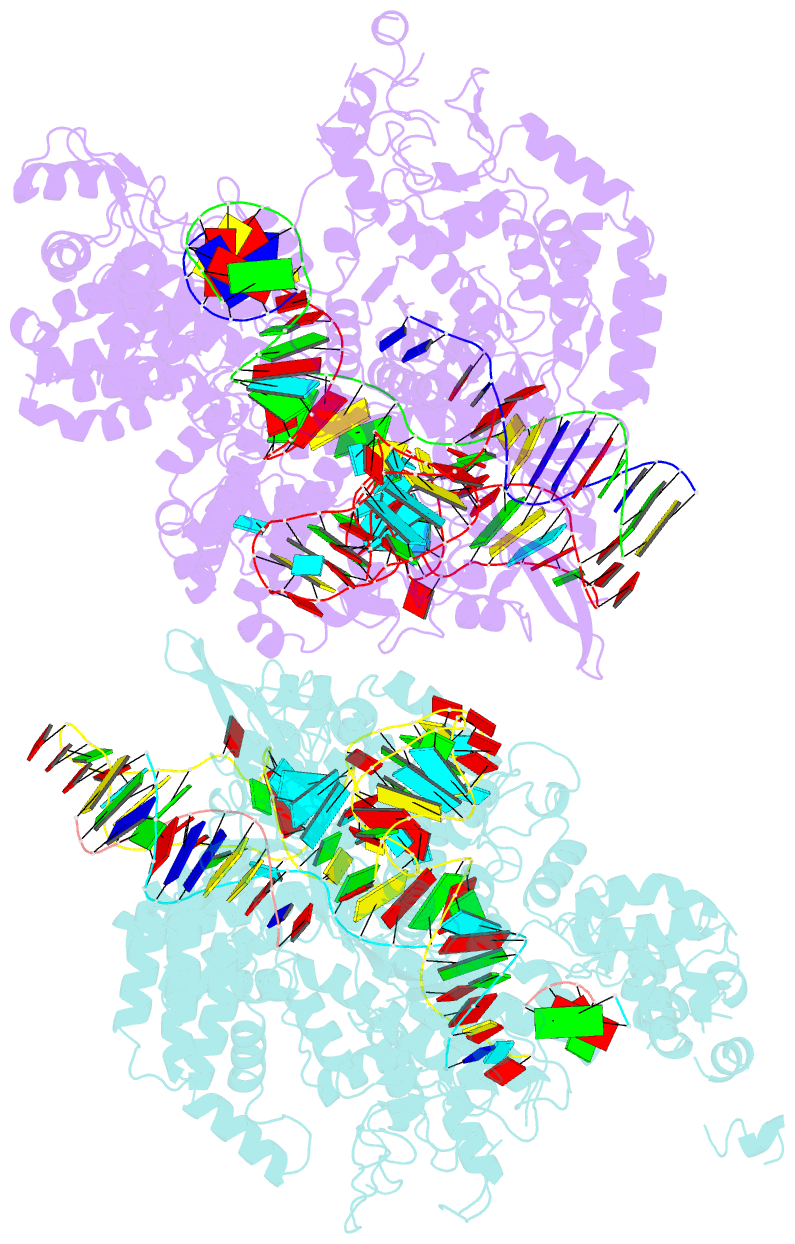
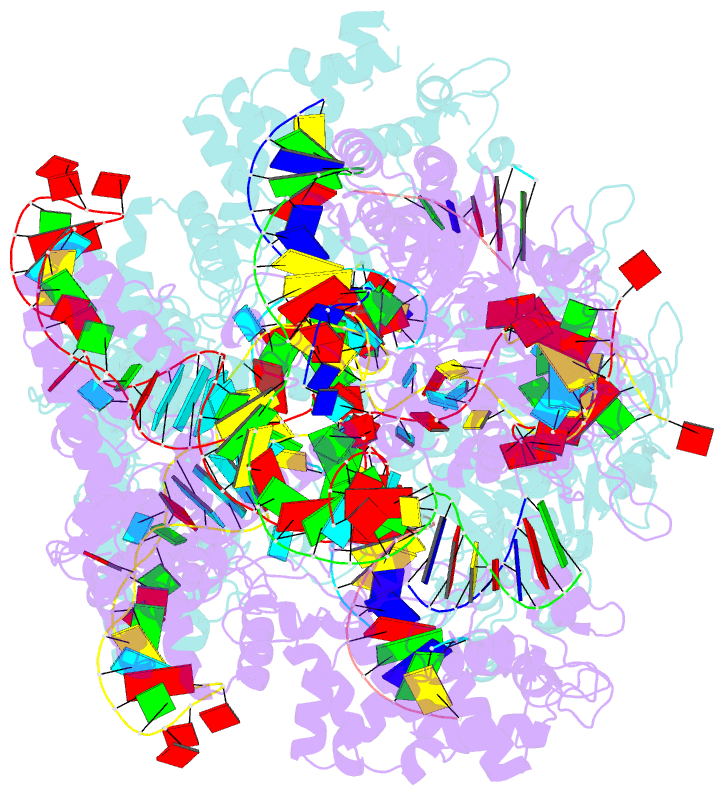
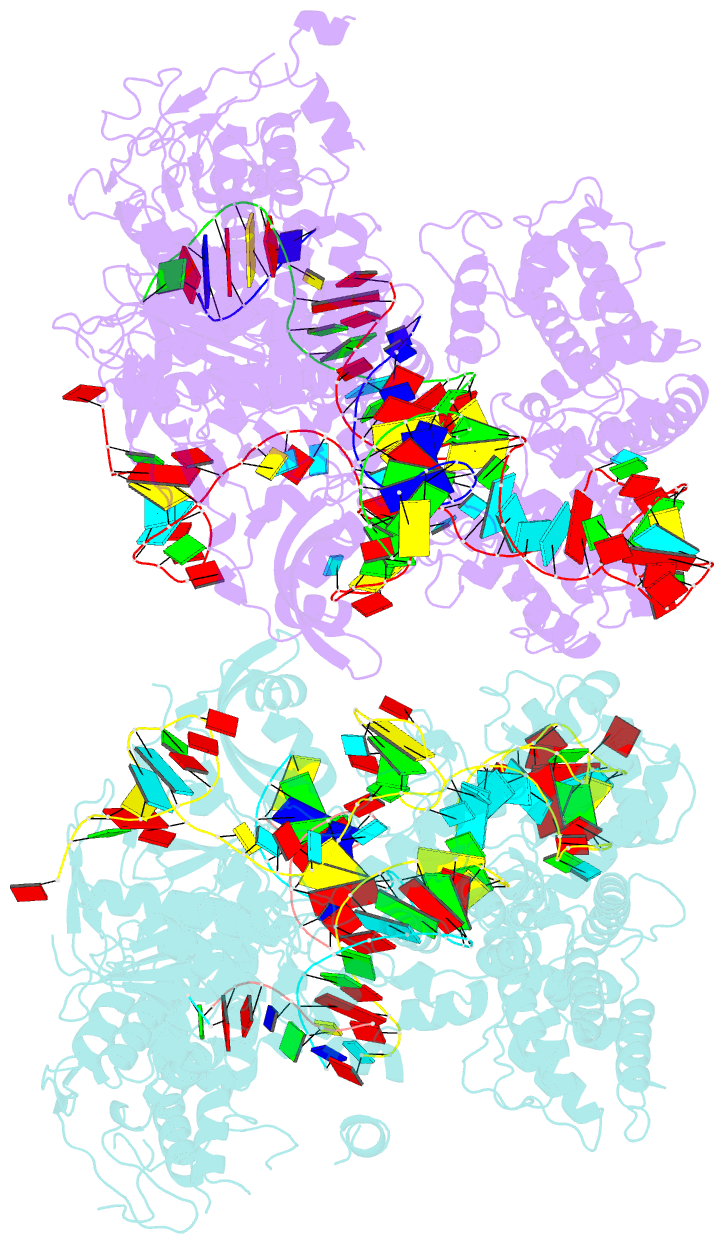
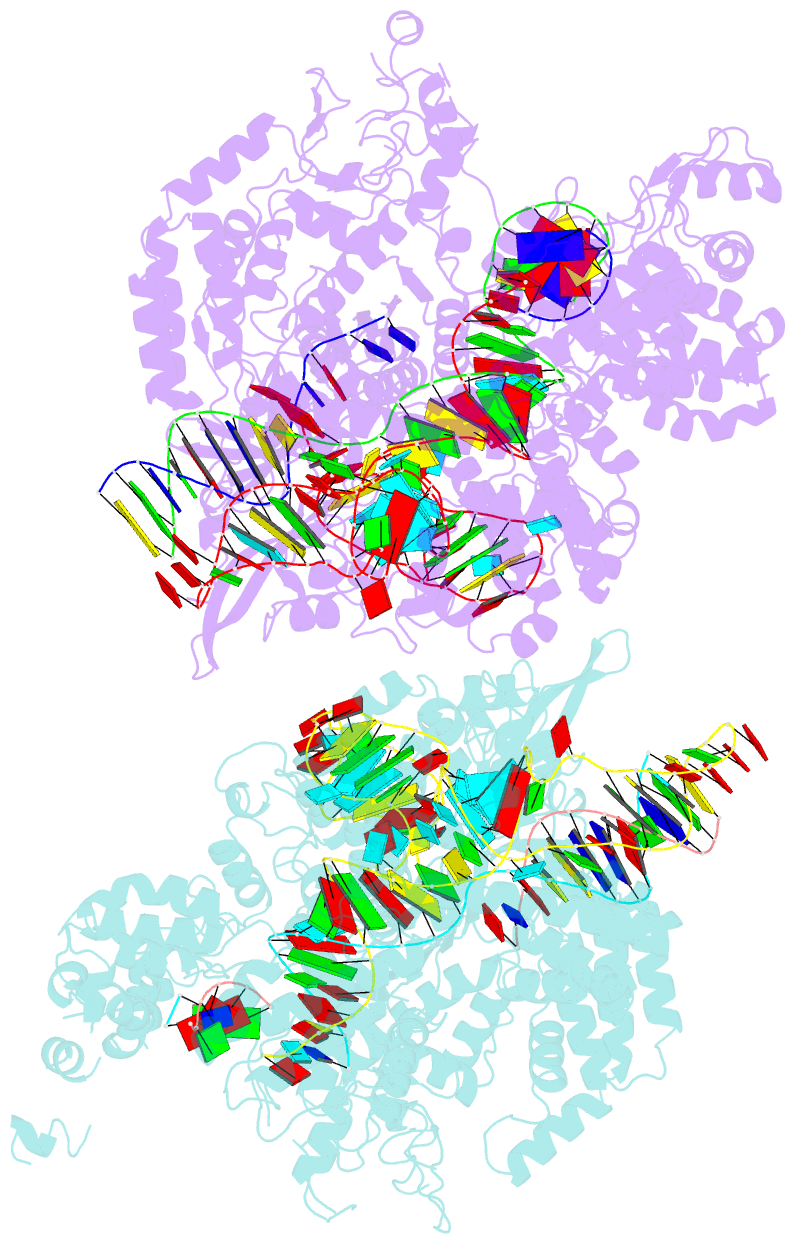
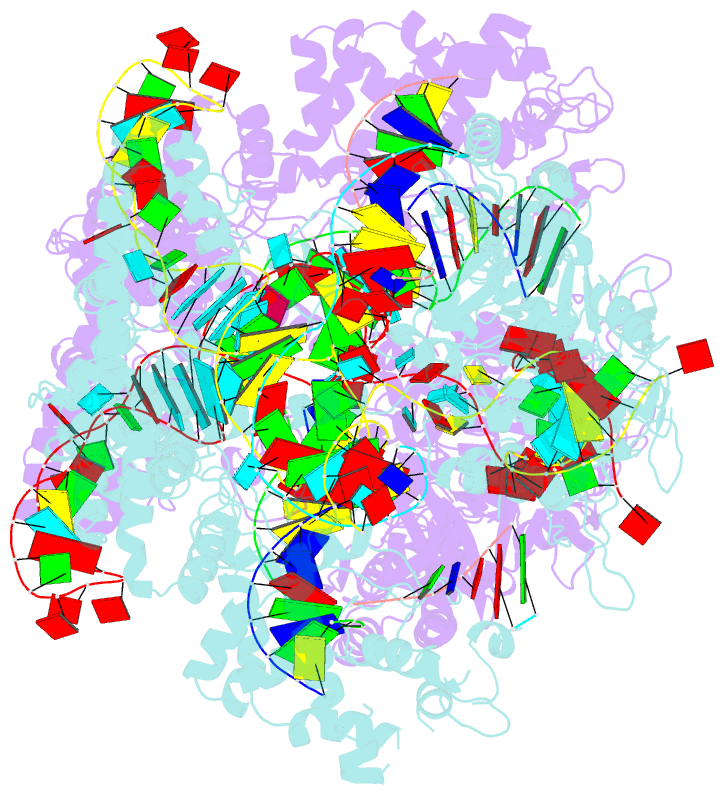
Summary information and primary citation
- PDB-id
- 7z4d; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase
- Method
- X-ray (3.1 Å)
- Summary
- Crystal structure of spcas9 bound to a 10 nucleotide complementary DNA substrate
- Reference
- Pacesa M, Loeff L, Querques I, Muckenfuss LM, Sawicka M, Jinek M (2022): "R-loop formation and conformational activation mechanisms of Cas9." Nature, 609, 191-196. doi: 10.1038/s41586-022-05114-0.
- Abstract
- Cas9 is a CRISPR-associated endonuclease capable of RNA-guided, site-specific DNA cleavage1-3. The programmable activity of Cas9 has been widely utilized for genome editing applications4-6, yet its precise mechanisms of target DNA binding and off-target discrimination remain incompletely understood. Here we report a series of cryo-electron microscopy structures of Streptococcus pyogenes Cas9 capturing the directional process of target DNA hybridization. In the early phase of R-loop formation, the Cas9 REC2 and REC3 domains form a positively charged cleft that accommodates the distal end of the target DNA duplex. Guide-target hybridization past the seed region induces rearrangements of the REC2 and REC3 domains and relocation of the HNH nuclease domain to assume a catalytically incompetent checkpoint conformation. Completion of the guide-target heteroduplex triggers conformational activation of the HNH nuclease domain, enabled by distortion of the guide-target heteroduplex, and complementary REC2 and REC3 domain rearrangements. Together, these results establish a structural framework for target DNA-dependent activation of Cas9 that sheds light on its conformational checkpoint mechanism and may facilitate the development of novel Cas9 variants and guide RNA designs with enhanced specificity and activity.