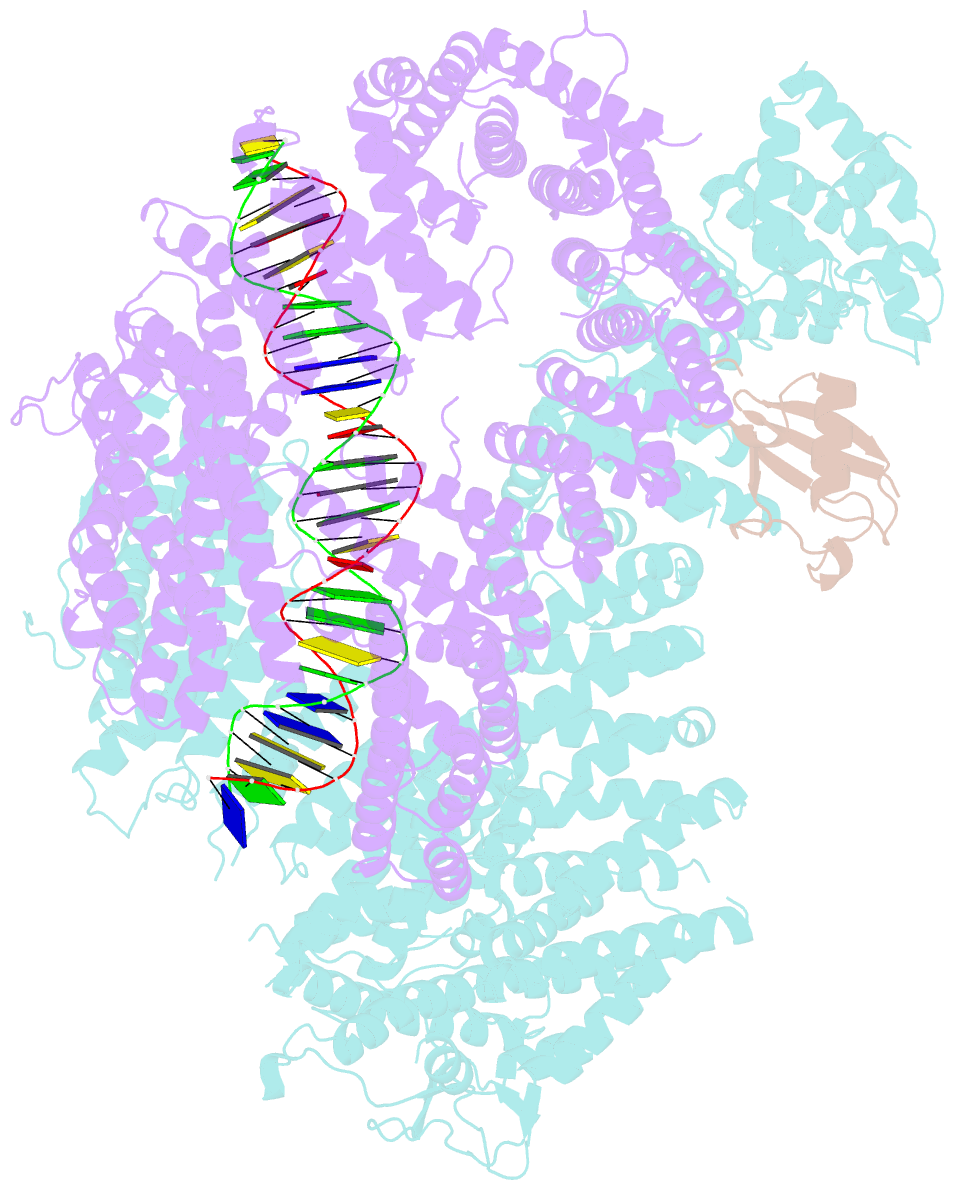
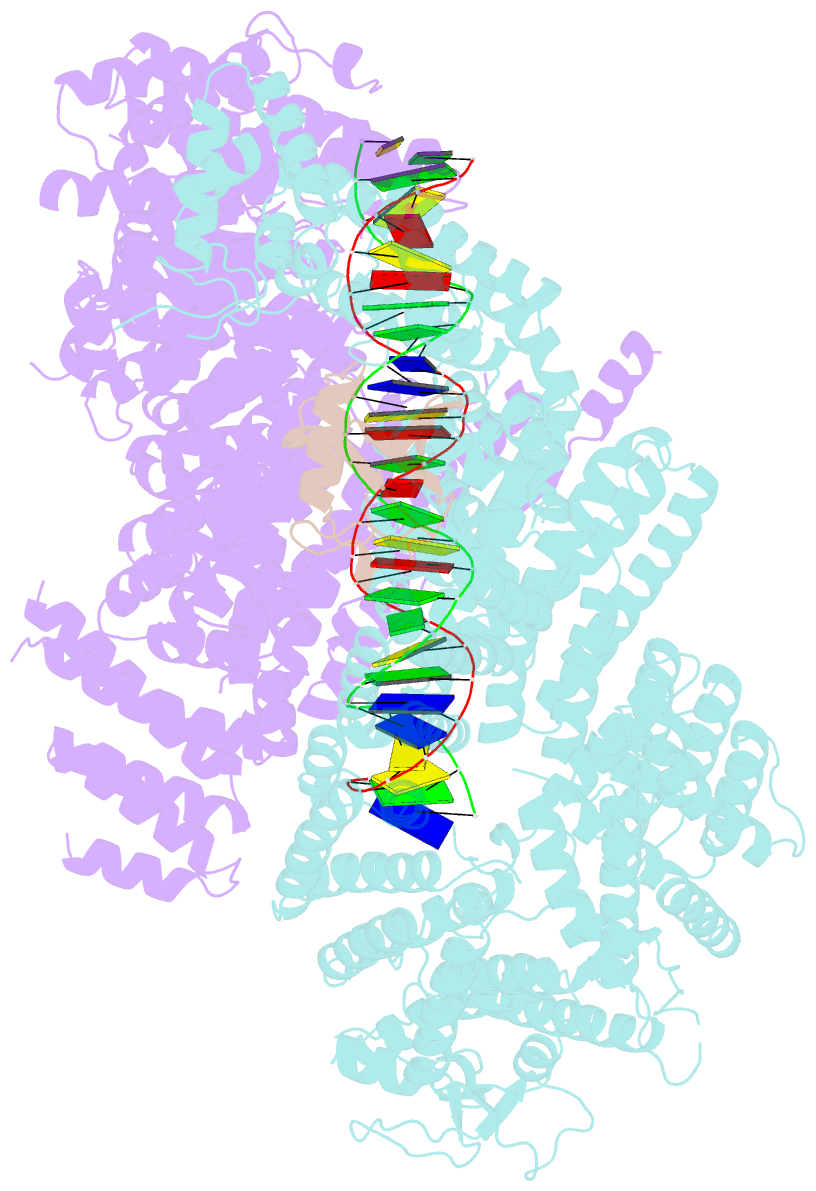
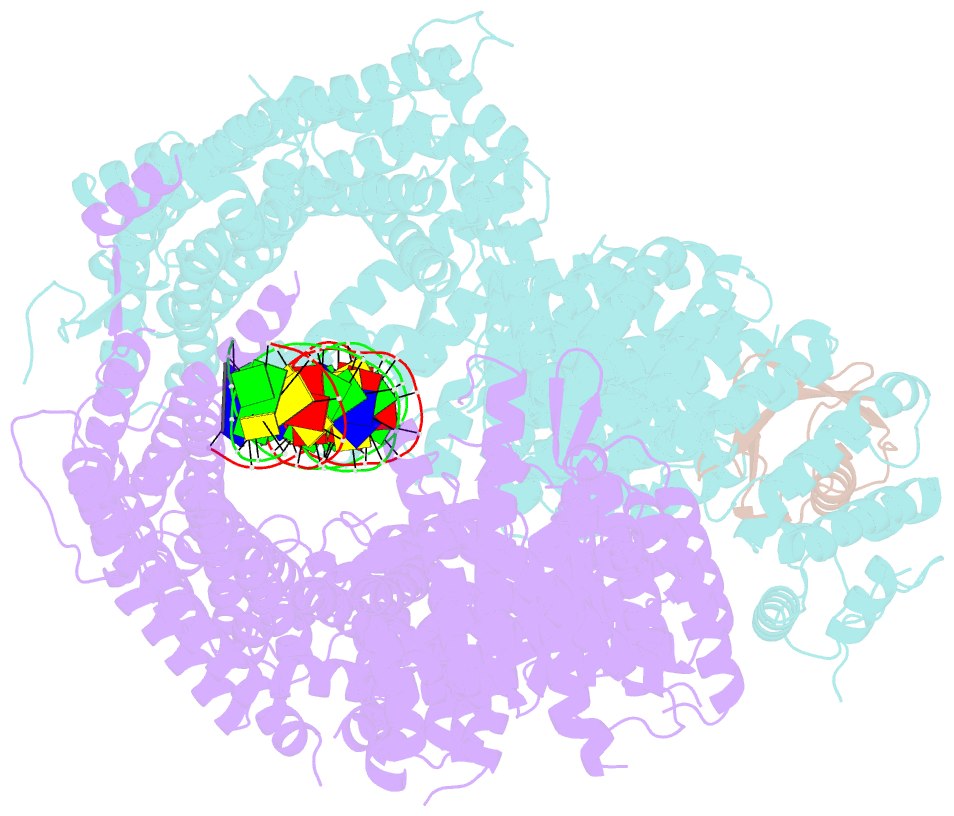
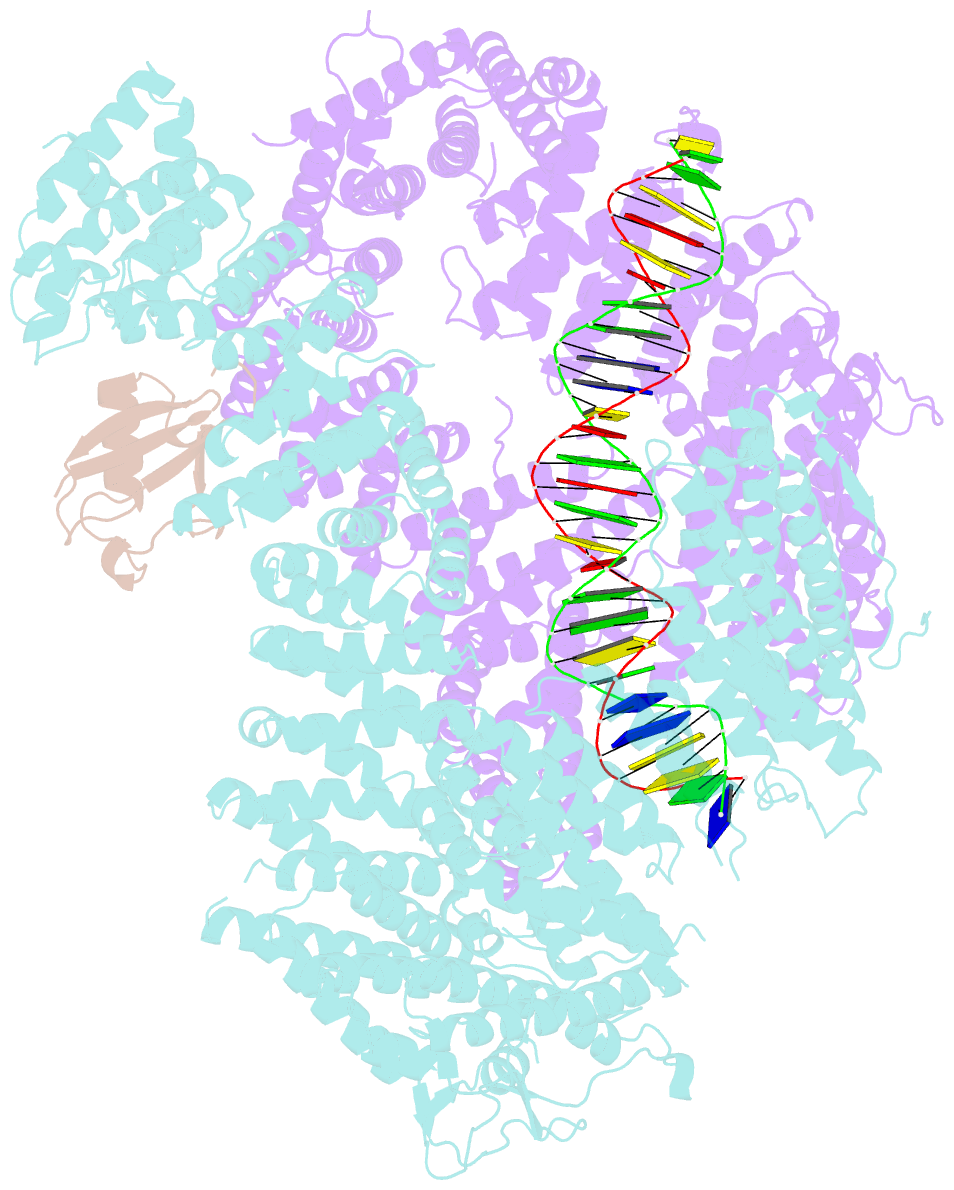
Summary information and primary citation
- PDB-id
- 7zf1; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- cryo-EM (4.14 Å)
- Summary
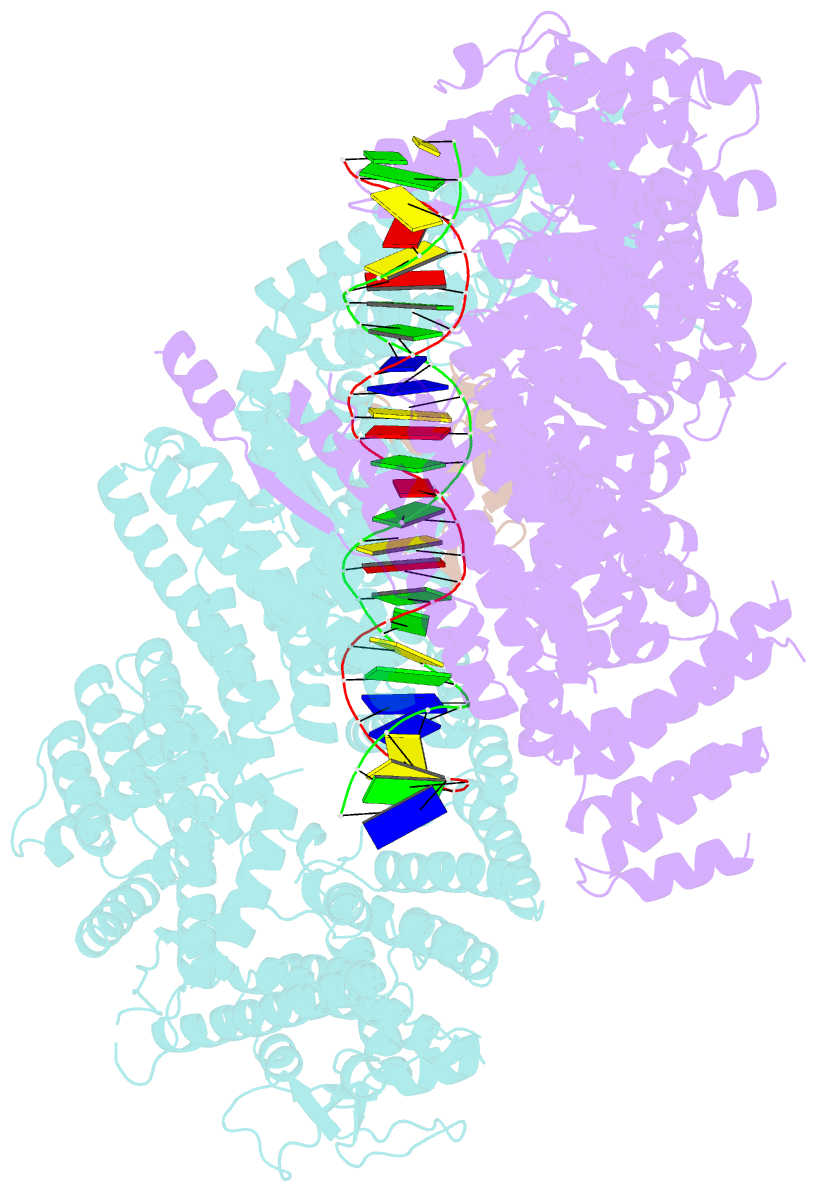
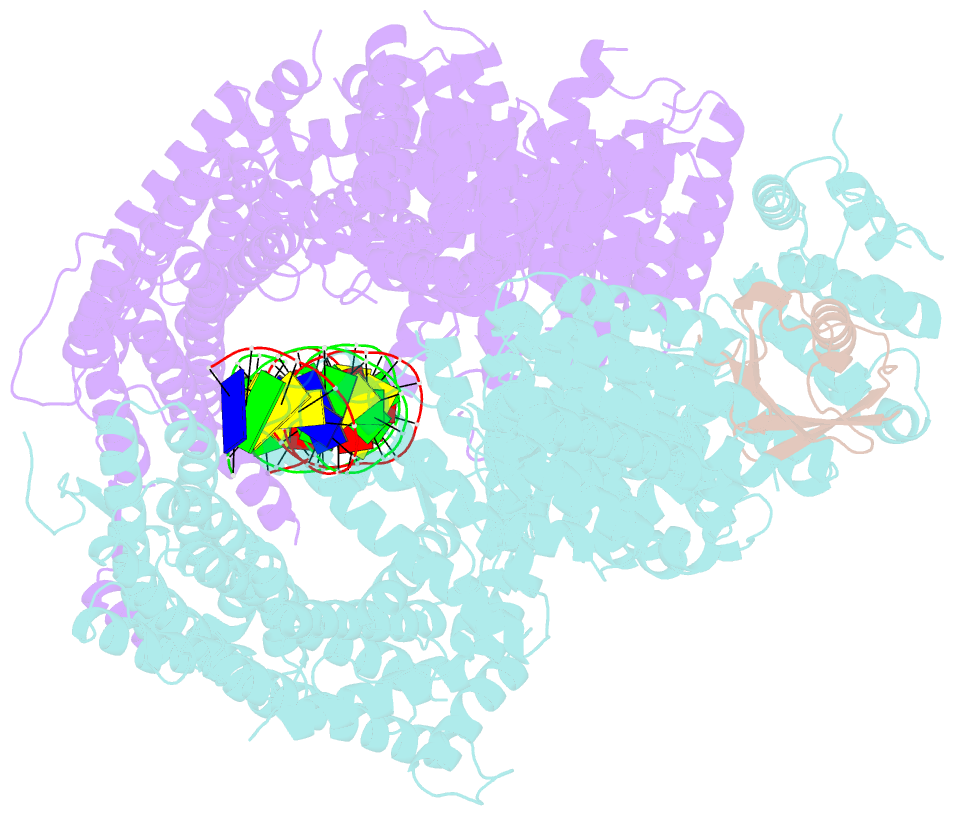
- Structure of ubiquitinated fanci in complex with fancd2 and double-stranded DNA
- Reference
- Lemonidis K, Rennie ML, Arkinson C, Chaugule VK, Clarke M, Streetley J, Walden H (2023): "Structural and biochemical basis of interdependent FANCI-FANCD2 ubiquitination." Embo J., 42, e111898. doi: 10.15252/embj.2022111898.
- Abstract
- Di-monoubiquitination of the FANCI-FANCD2 (ID2) complex is a central and crucial step for the repair of DNA interstrand crosslinks via the Fanconi anaemia pathway. While FANCD2 ubiquitination precedes FANCI ubiquitination, FANCD2 is also deubiquitinated at a faster rate than FANCI, which can result in a FANCI-ubiquitinated ID2 complex (IUb D2). Here, we present a 4.1 Å cryo-EM structure of IUb D2 complex bound to double-stranded DNA. We show that this complex, like ID2Ub and IUb D2Ub , is also in the closed ID2 conformation and clamps on DNA. The target lysine of FANCD2 (K561) becomes fully exposed in the IUb D2-DNA structure and is thus primed for ubiquitination. Similarly, FANCI's target lysine (K523) is also primed for ubiquitination in the ID2Ub -DNA complex. The IUb D2-DNA complex exhibits deubiquitination resistance, conferred by the presence of DNA and FANCD2. ID2Ub -DNA, on the other hand, can be efficiently deubiquitinated by USP1-UAF1, unless further ubiquitination on FANCI occurs. Therefore, FANCI ubiquitination effectively maintains FANCD2 ubiquitination in two ways: it prevents excessive FANCD2 deubiquitination within an IUb D2Ub -DNA complex, and it enables re-ubiquitination of FANCD2 within a transient, closed-on-DNA, IUb D2 complex.