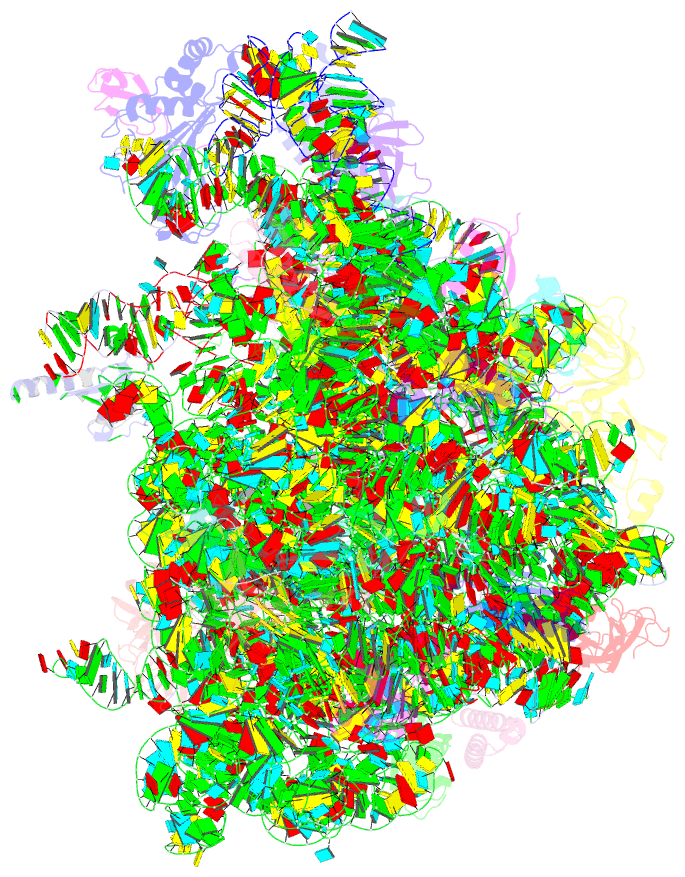
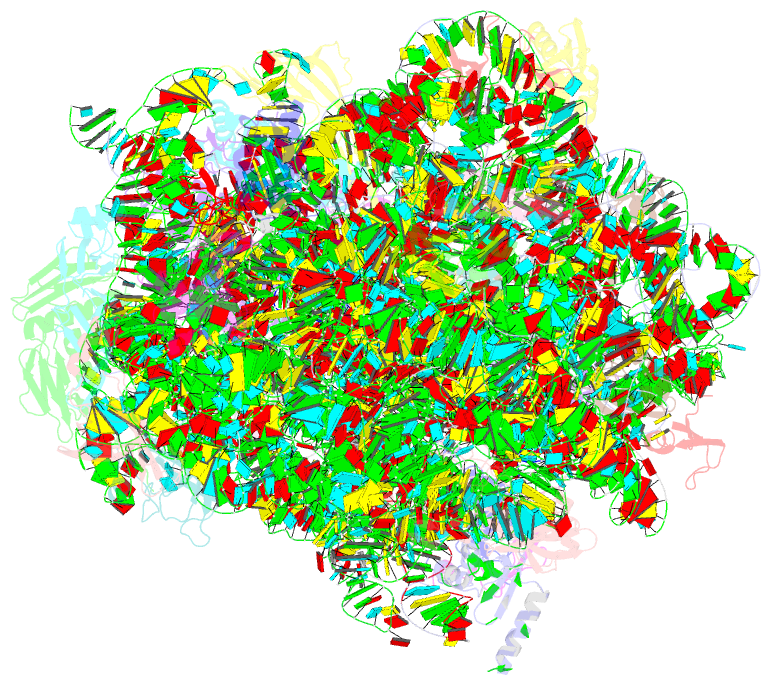
Summary information and primary citation
- PDB-id
- 8ap4; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- translation
- Method
- cryo-EM (3.0 Å)
- Summary
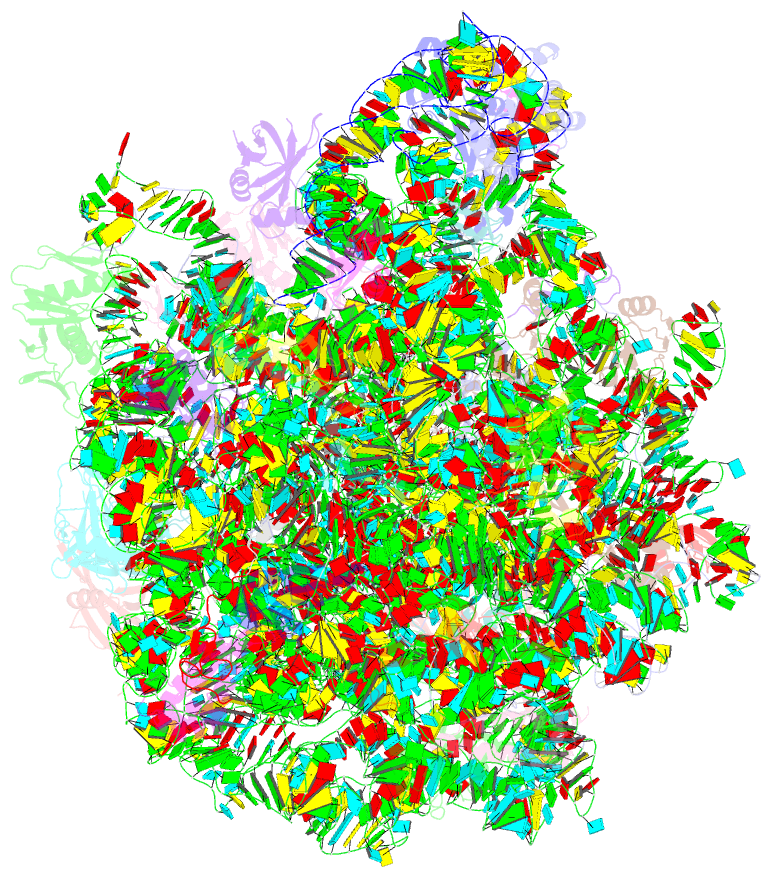
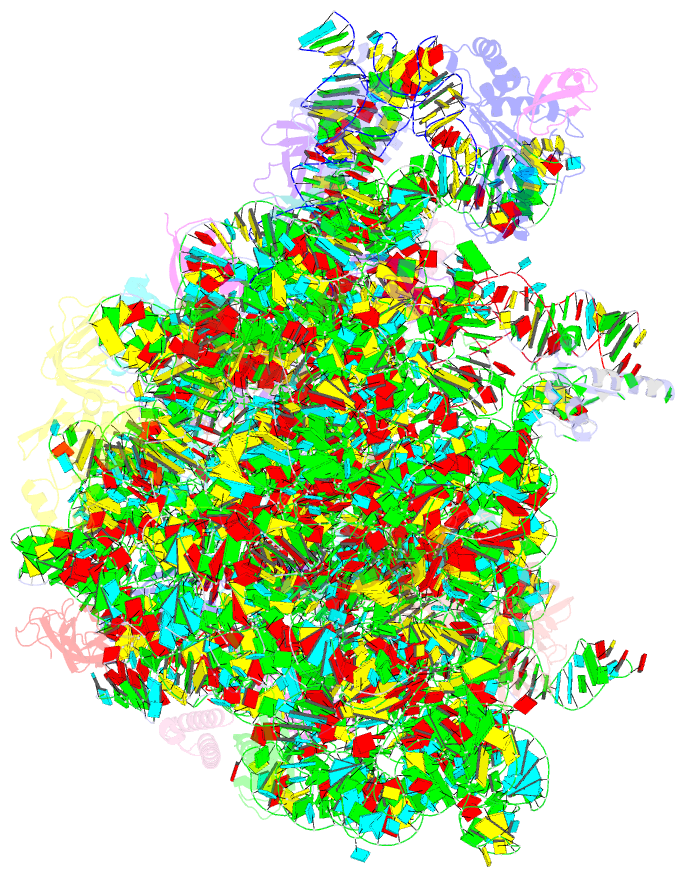
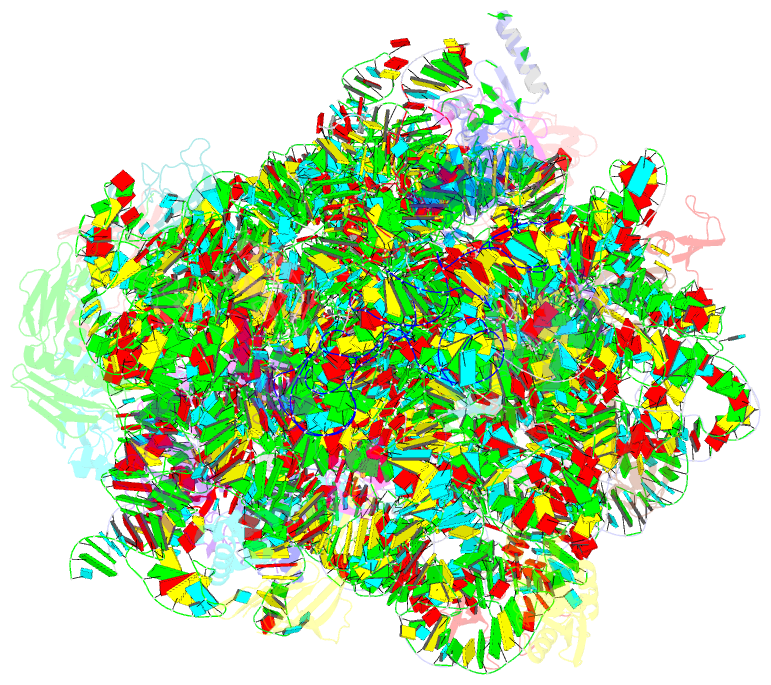
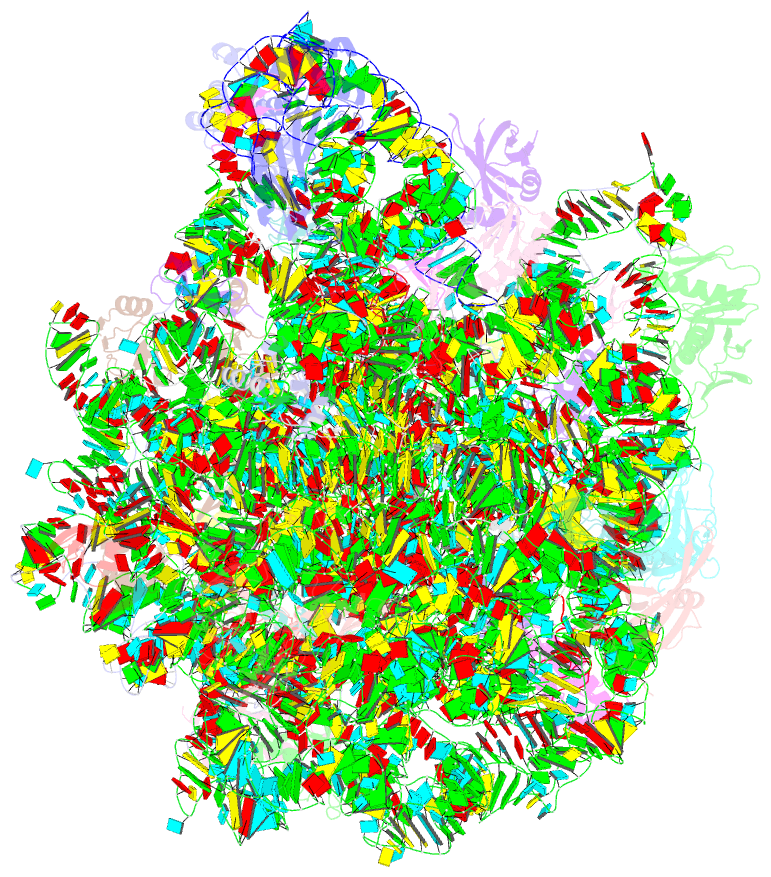
- Structure of escherischia coli heat shock protein hsp15 in complex with ribosomal 50s subunits bearing peptidyl-trna
- Reference
- Safdari HA, Kasvandik S, Polte C, Ignatova Z, Tenson T, Wilson DN (2022): "Structure of Escherichia coli heat shock protein Hsp15 in complex with the ribosomal 50S subunit bearing peptidyl-tRNA." Nucleic Acids Res., 50, 12515-12526. doi: 10.1093/nar/gkac1035.
- Abstract
- In Escherichia coli, the heat shock protein 15 (Hsp15) is part of the cellular response to elevated temperature. Hsp15 interacts with peptidyl-tRNA-50S complexes that arise upon dissociation of translating 70S ribosomes, and is proposed to facilitate their rescue and recycling. A previous structure of E. coli Hsp15 in complex with peptidyl-tRNA-50S complex reported a binding site located at the central protuberance of the 50S subunit. By contrast, recent structures of RqcP, the Hsp15 homolog in Bacillus subtilis, in complex with peptidyl-tRNA-50S complexes have revealed a distinct site positioned between the anticodon-stem-loop (ASL) of the P-site tRNA and H69 of the 23S rRNA. Here we demonstrate that exposure of E. coli cells to heat shock leads to a decrease in 70S ribosomes and accumulation of 50S subunits, thus identifying a natural substrate for Hsp15 binding. Additionally, we have determined a cryo-EM reconstruction of the Hsp15-50S-peptidyl-tRNA complex isolated from heat shocked E. coli cells, revealing that Hsp15 binds to the 50S-peptidyl-tRNA complex analogously to its B. subtilis homolog RqcP. Collectively, our findings support a model where Hsp15 stabilizes the peptidyl-tRNA in the P-site and thereby promotes access to the A-site for putative rescue factors to release the aberrant nascent polypeptide chain.