Summary information and primary citation
- PDB-id
- 8axf; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- viral protein
- Method
- X-ray (2.54 Å)
- Summary
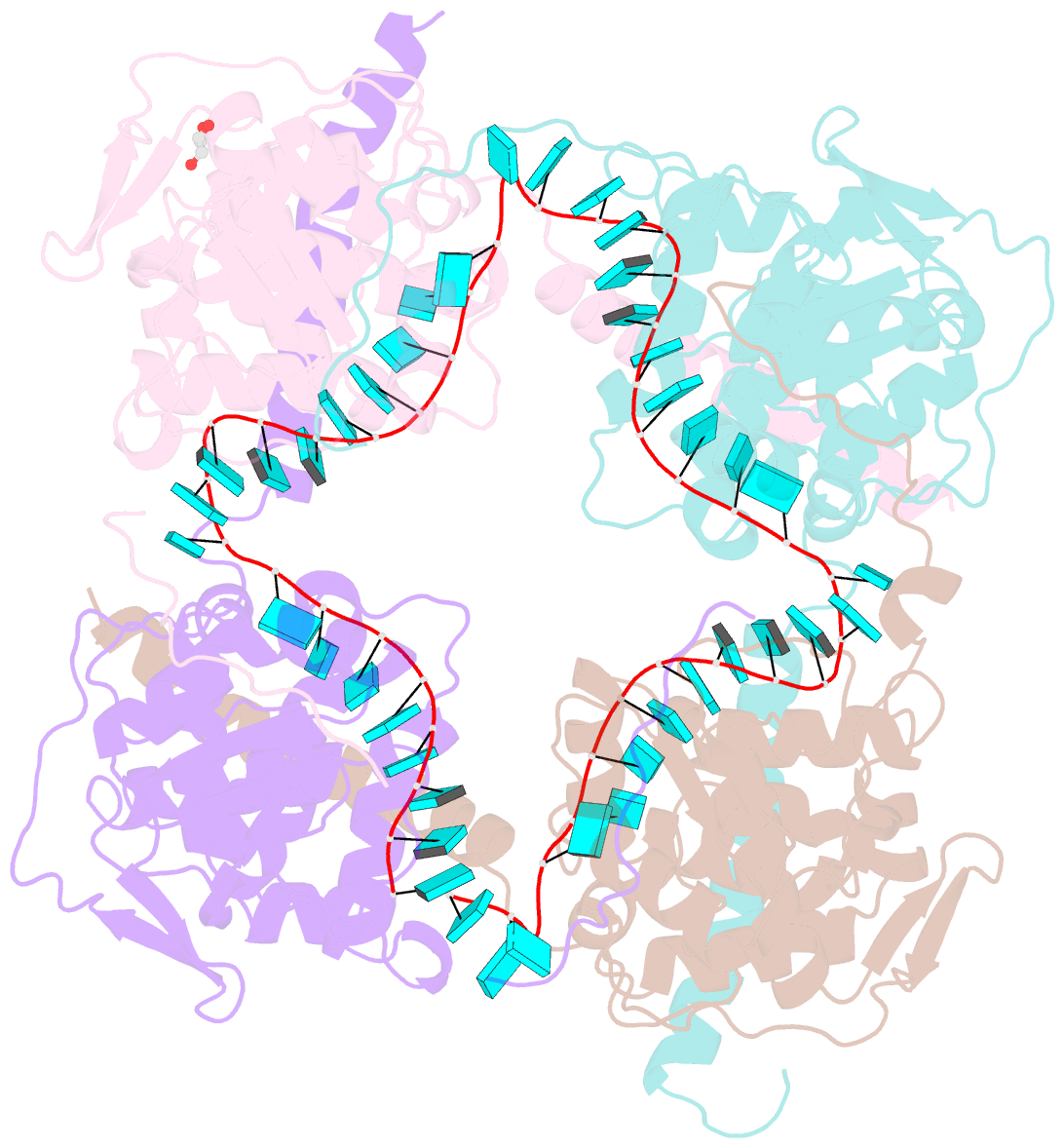
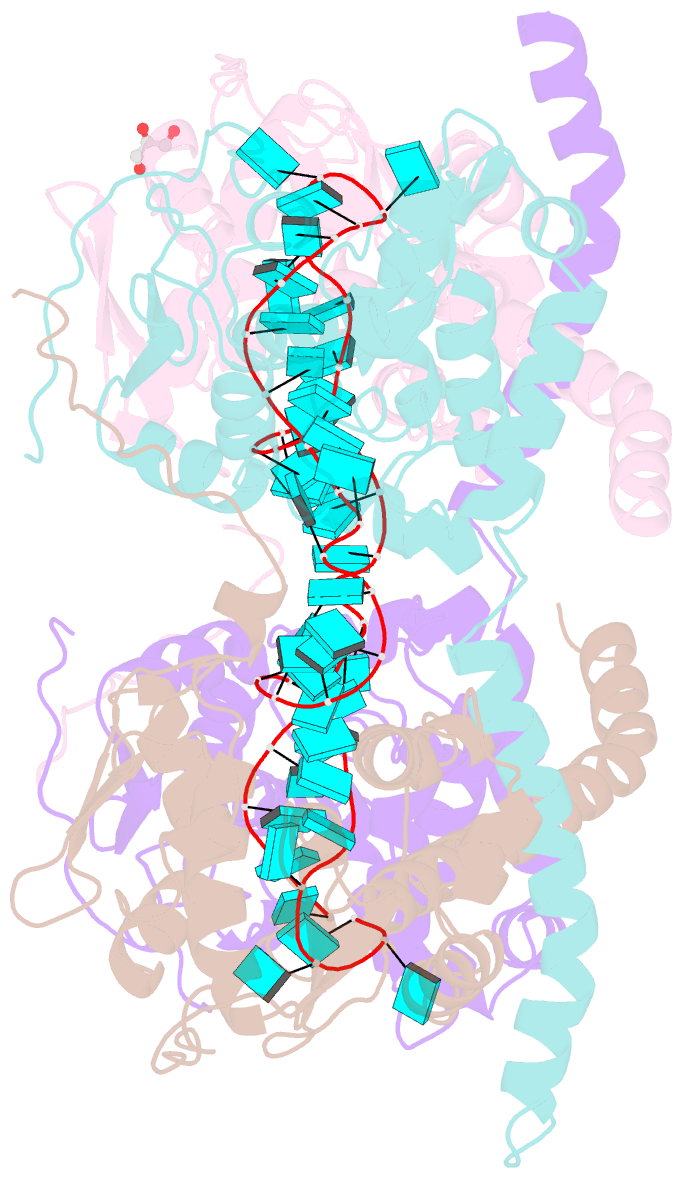
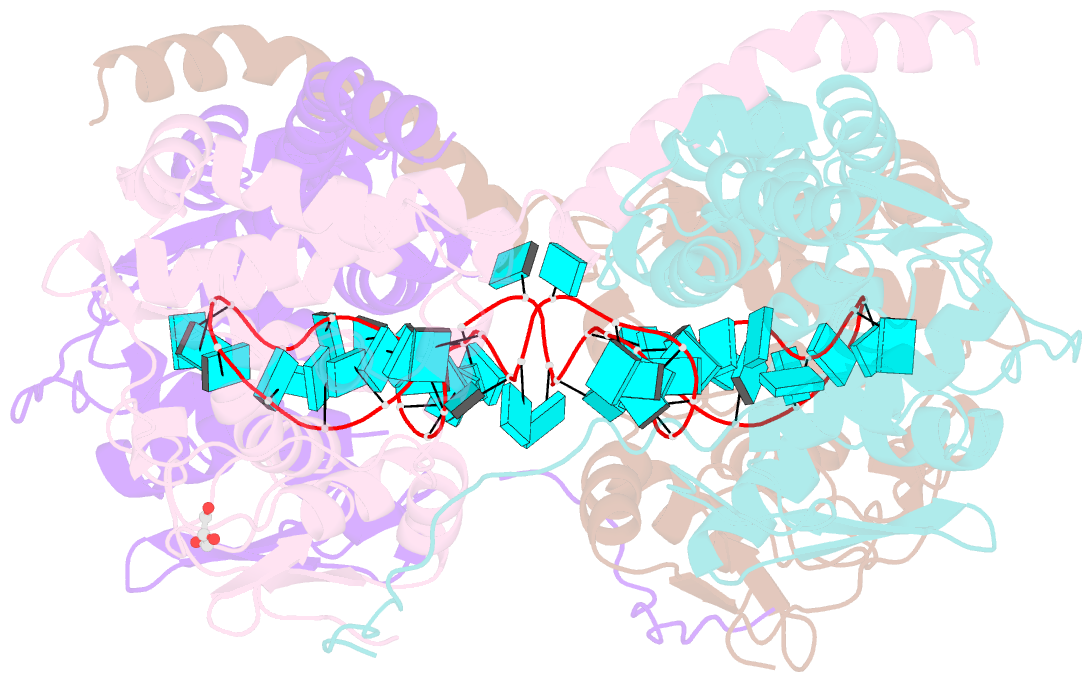
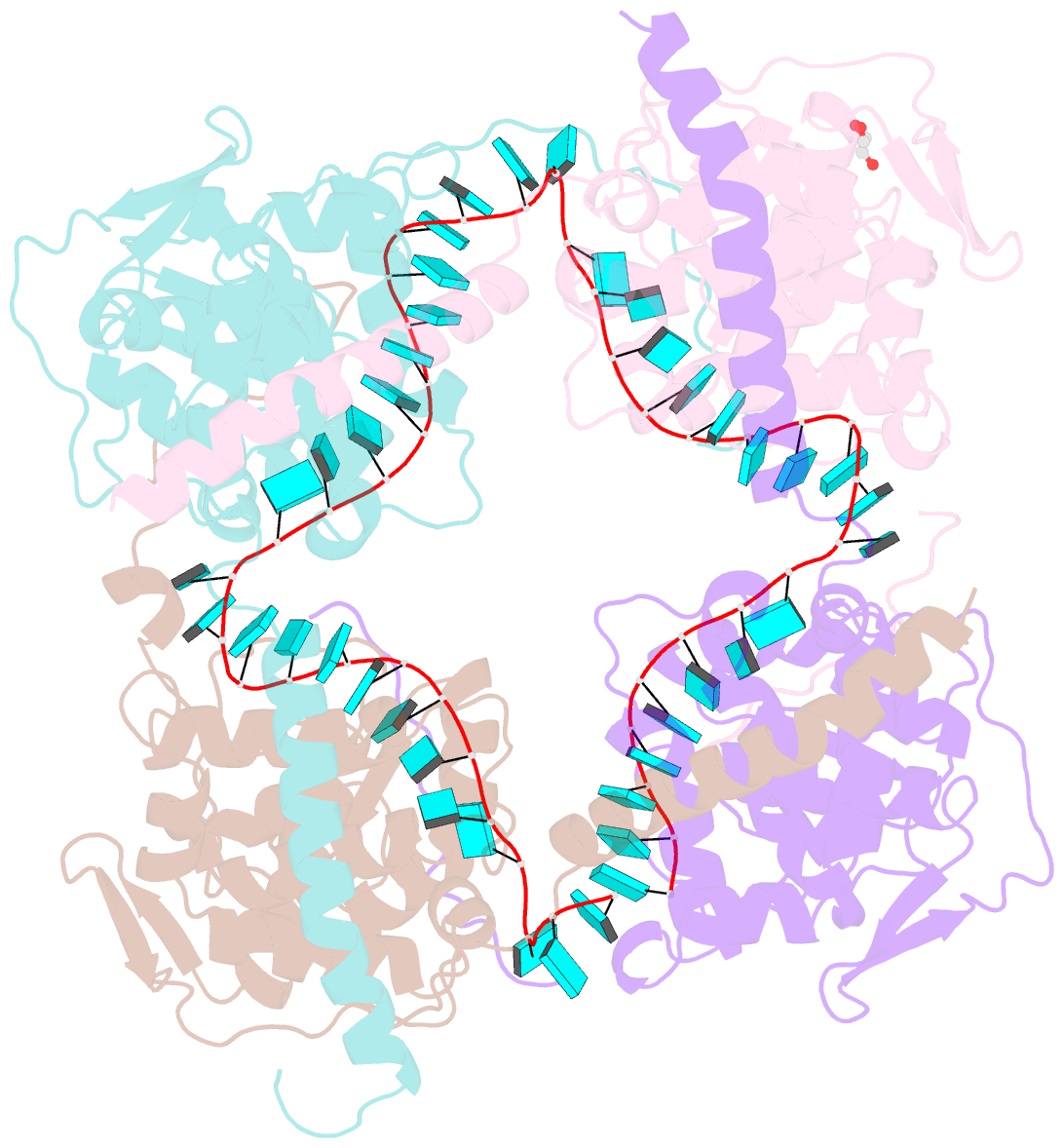
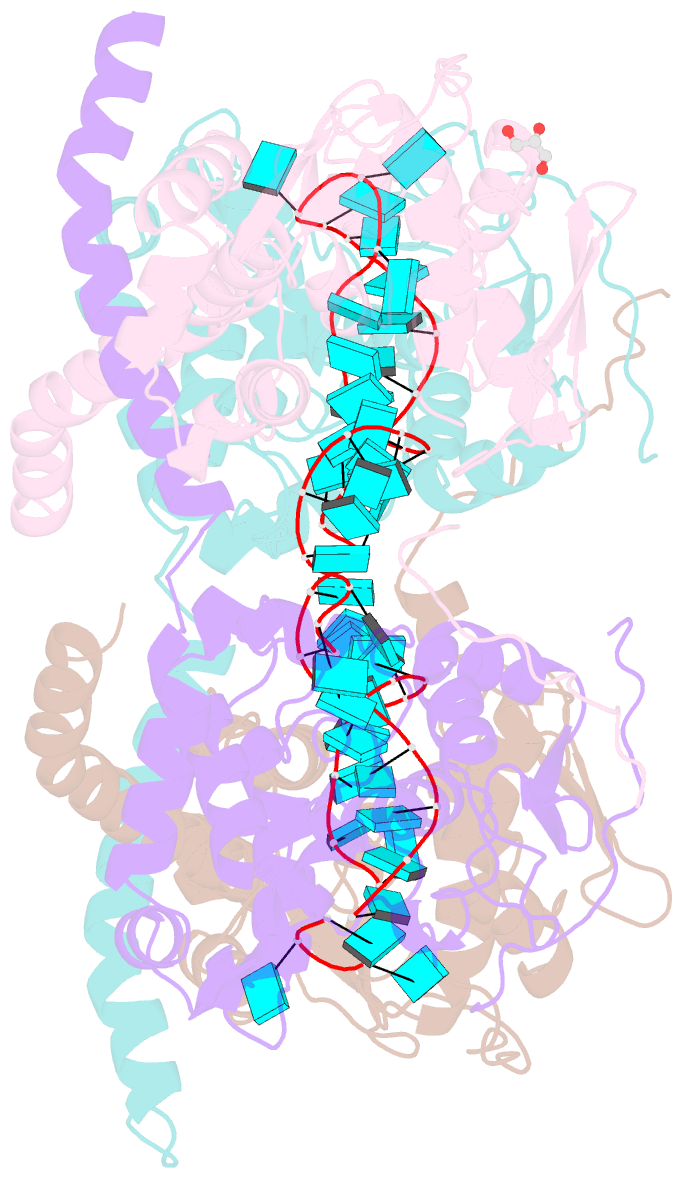
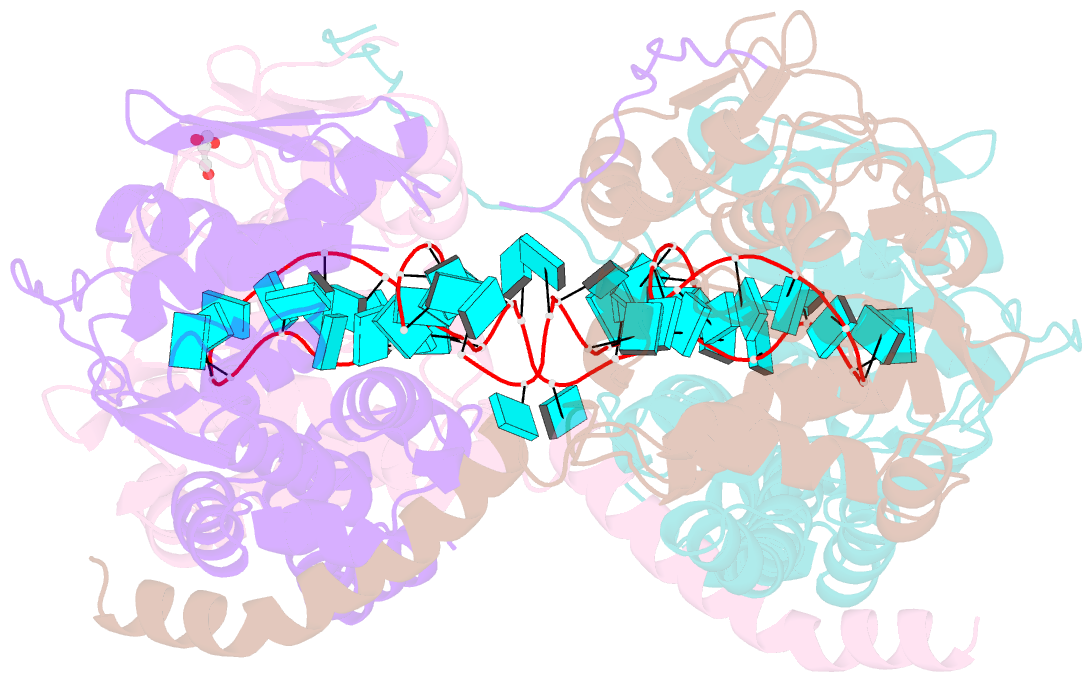
- Crystal structure of fmv n bound to 42-mer ssrna
- Reference
- Izhaki-Tavor LS, Yechezkel IG, Alter J, Dessau M (2023): "RNA Encapsulation Mode and Evolutionary Insights from the Crystal Structure of Emaravirus Nucleoprotein." Microbiol Spectr, 11, e0501822. doi: 10.1128/spectrum.05018-22.
- Abstract
- Enveloped RNA viruses are rare among plant viruses. Fimoviridae is a newly founded family of plant viruses within the Bunyavirales order that inflicts diverse crop losses worldwide. The fig mosaic virus (FMV), the representative member of the Fimoviridae family, was shown to be a causative agent for the fig mosaic disease. Like all bunyaviruses, FMV has a segmented, negative-sense, single-stranded RNA (ssRNA) genome that is encapsulated by the viral nucleoprotein (N). Here, we present high-resolution crystal structures of FMV N in its RNA-free and RNA-bound forms, revealing a "paper fortune teller" structural transition between the two states. The tightly packed tetramer of FNV N is similar to the structures of other N proteins of different members of the Bunyavirales order. In its RNA-bound form, the tetramer reorganizes to adopt a more open state that allows the accommodation of the RNA. Despite the low sequence similarity to N proteins of animal-infecting bunyaviruses, there is a striking structural resemblance between FMV N and nucleoproteins from members of the Peribunyaviridae, an animal-infecting family of viruses. This structural homology implies that enveloped plant viruses and animal-infecting viruses might have a common ancestor from which they diverged.