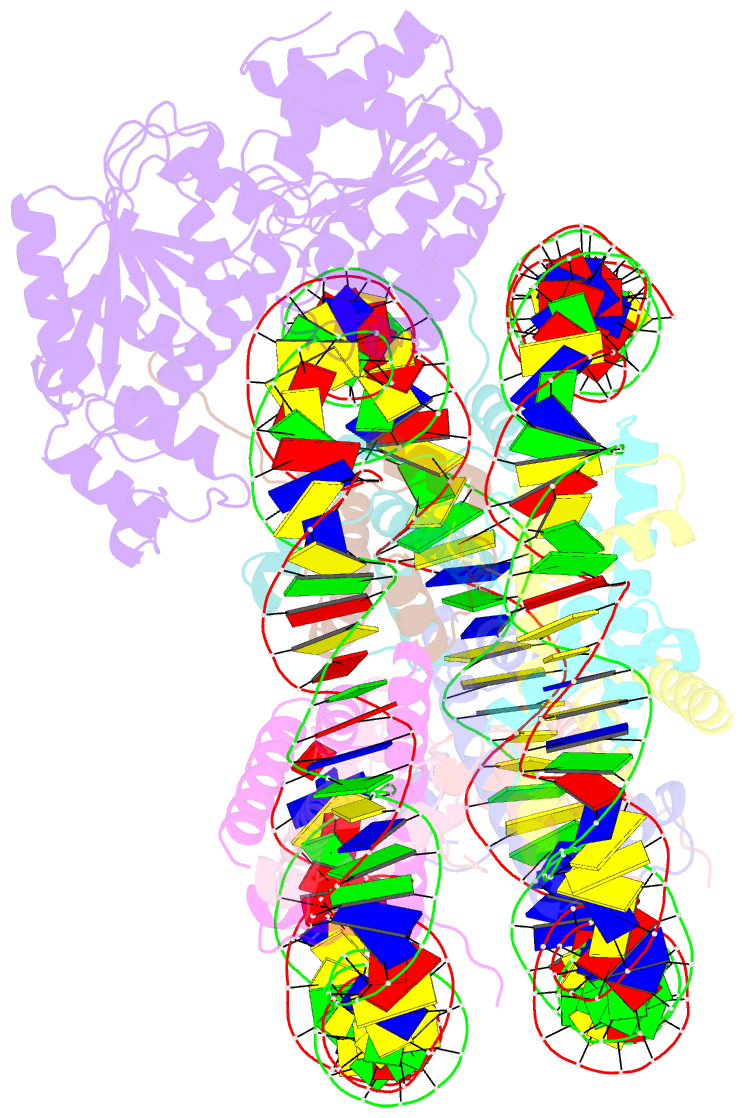
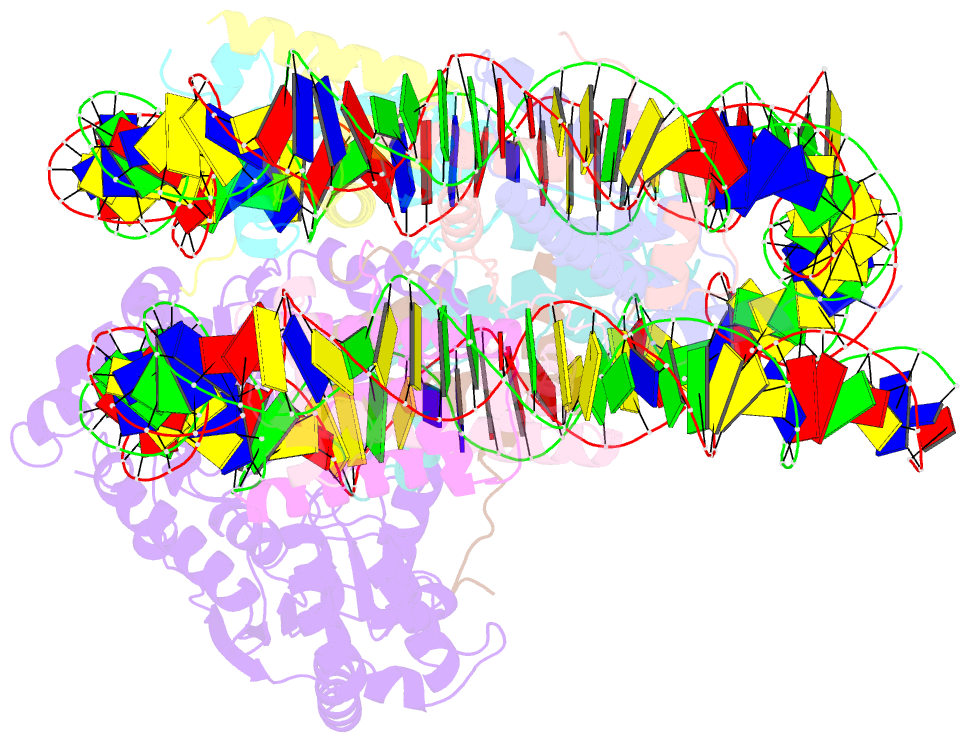
Summary information and primary citation
- PDB-id
- 8b0a; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- cryo-EM (3.0 Å)
- Summary
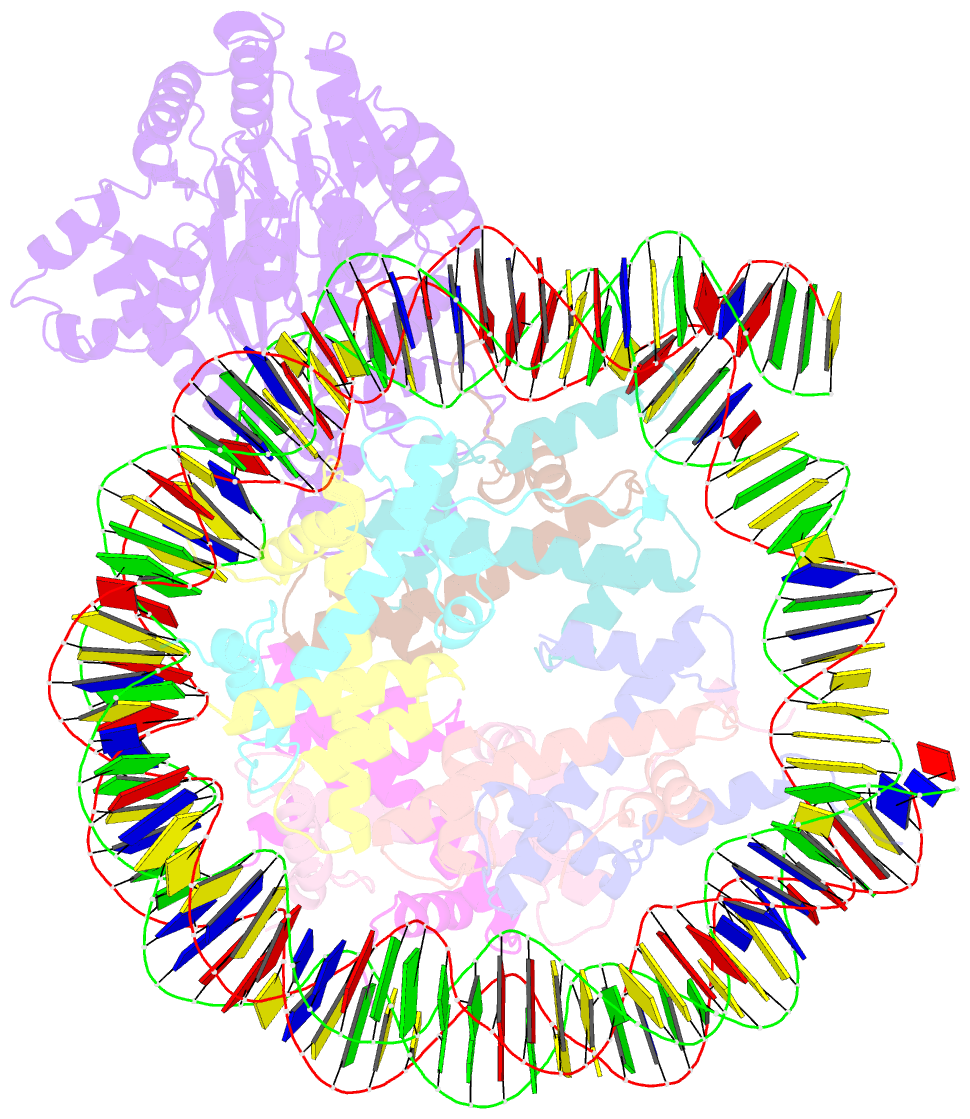
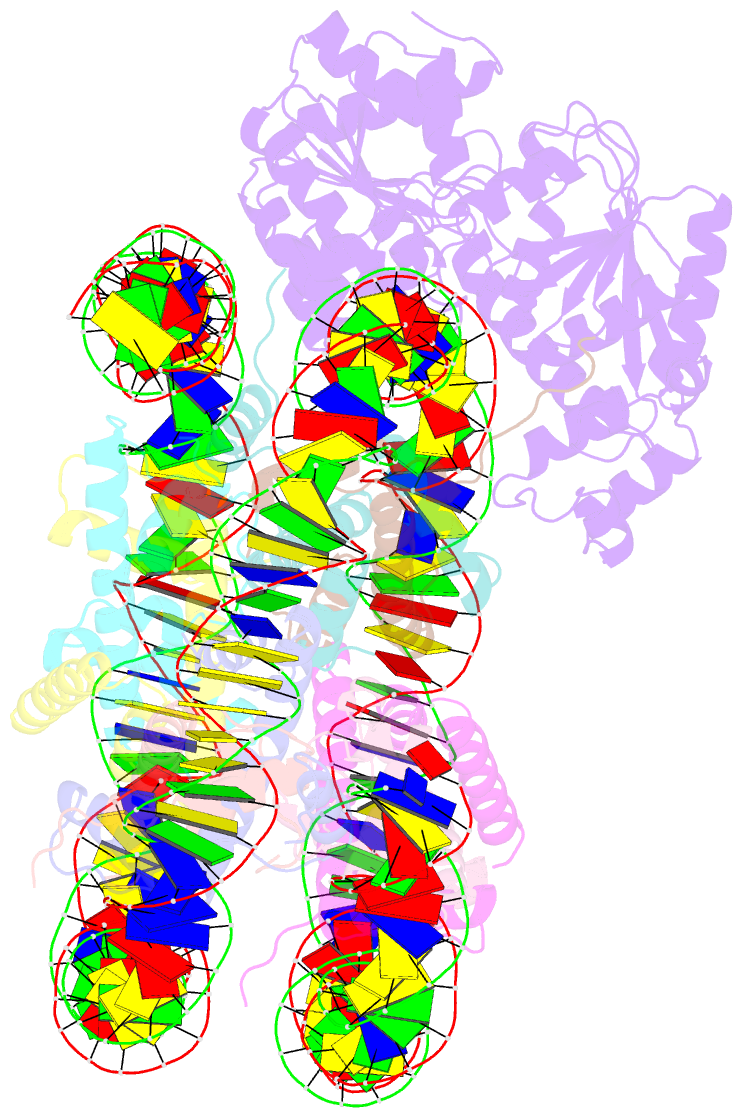
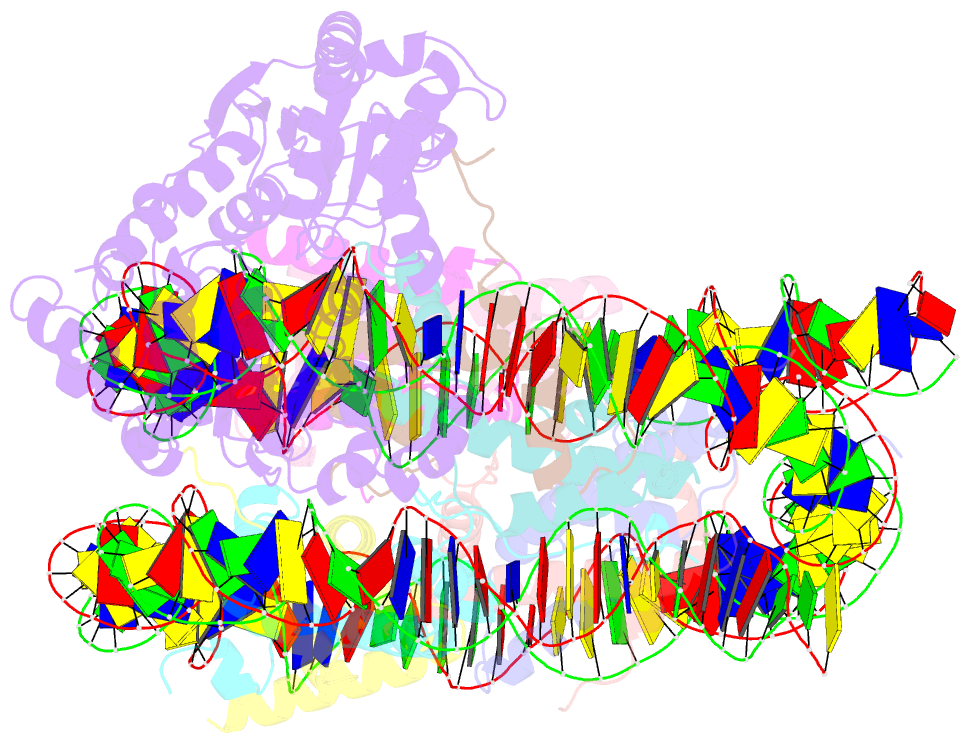
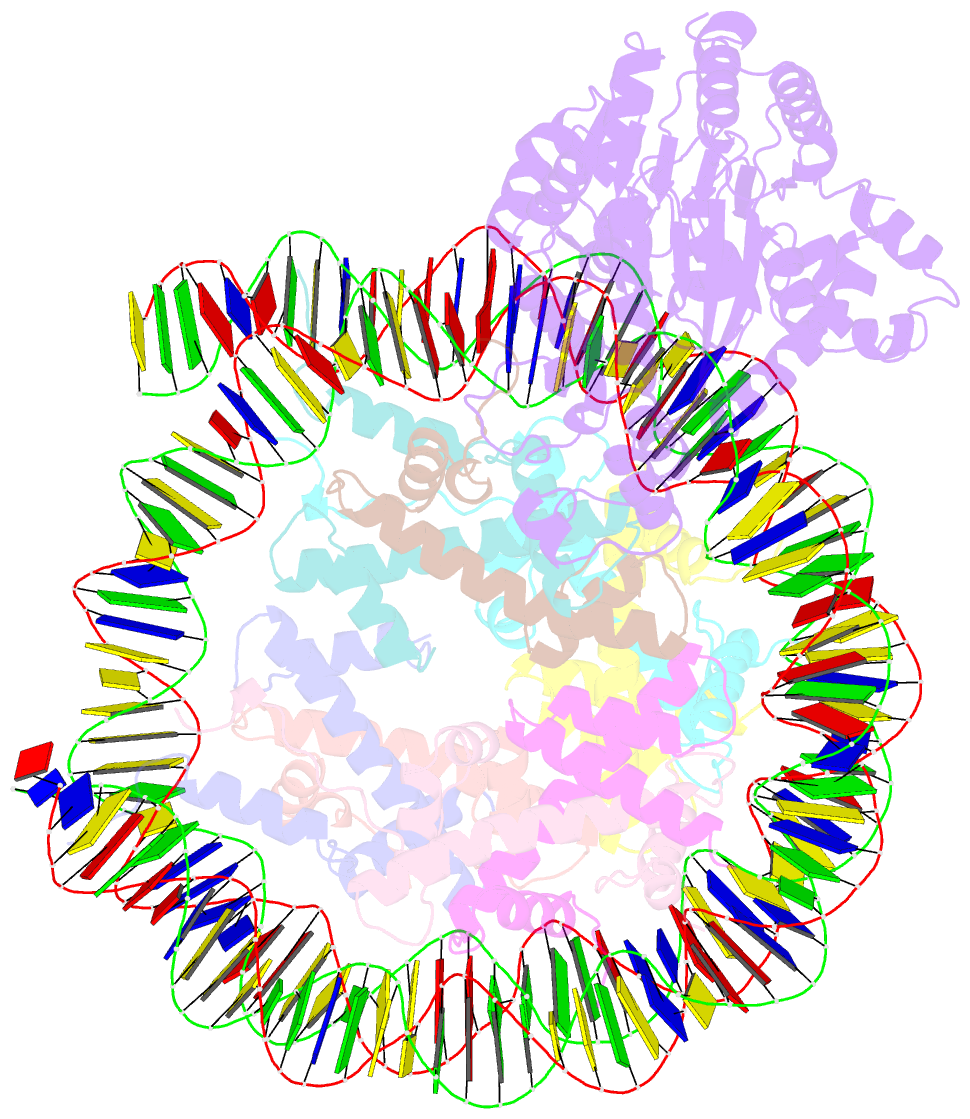
- cryo-EM structure of alc1 bound to an asymmetric, site-specifically parylated nucleosome
- Reference
- Bacic L, Gaullier G, Mohapatra J, Mao G, Brackmann K, Panfilov M, Liszczak G, Sabantsev A, Deindl S (2024): "Asymmetric nucleosome PARylation at DNA breaks mediates directional nucleosome sliding by ALC1." Nat Commun, 15, 1000. doi: 10.1038/s41467-024-45237-8.
- Abstract
- The chromatin remodeler ALC1 is activated by DNA damage-induced poly(ADP-ribose) deposited by PARP1/PARP2 and their co-factor HPF1. ALC1 has emerged as a cancer drug target, but how it is recruited to ADP-ribosylated nucleosomes to affect their positioning near DNA breaks is unknown. Here we find that PARP1/HPF1 preferentially initiates ADP-ribosylation on the histone H2B tail closest to the DNA break. To dissect the consequences of such asymmetry, we generate nucleosomes with a defined ADP-ribosylated H2B tail on one side only. The cryo-electron microscopy structure of ALC1 bound to such an asymmetric nucleosome indicates preferential engagement on one side. Using single-molecule FRET, we demonstrate that this asymmetric recruitment gives rise to directed sliding away from the DNA linker closest to the ADP-ribosylation site. Our data suggest a mechanism by which ALC1 slides nucleosomes away from a DNA break to render it more accessible to repair factors.