Summary information and primary citation
- PDB-id
- 8b0r; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase
- Method
- X-ray (2.2 Å)
- Summary
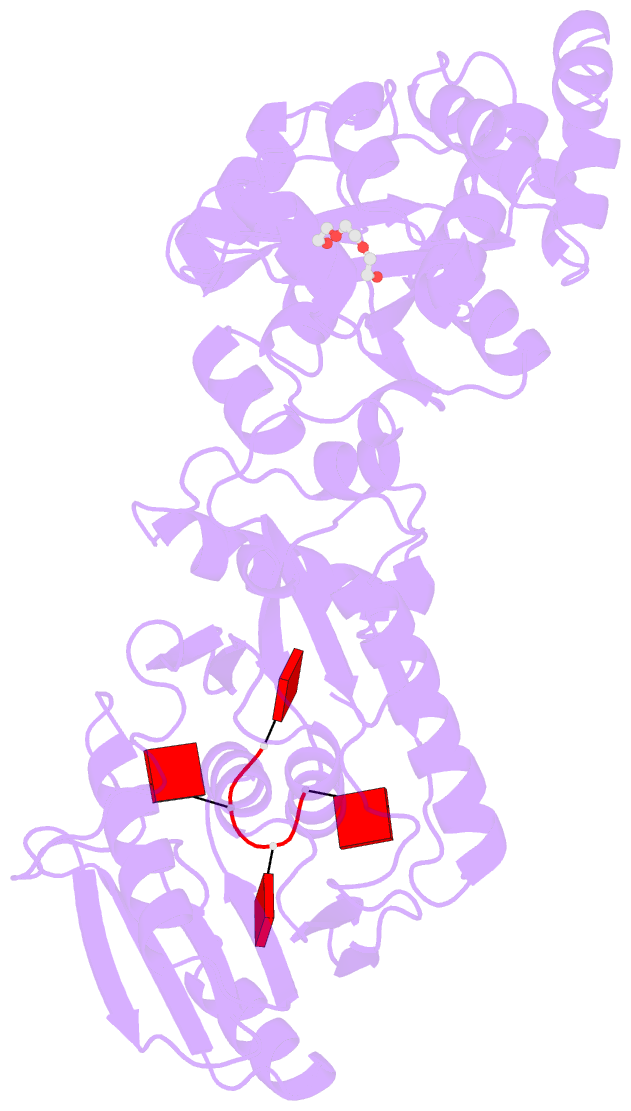
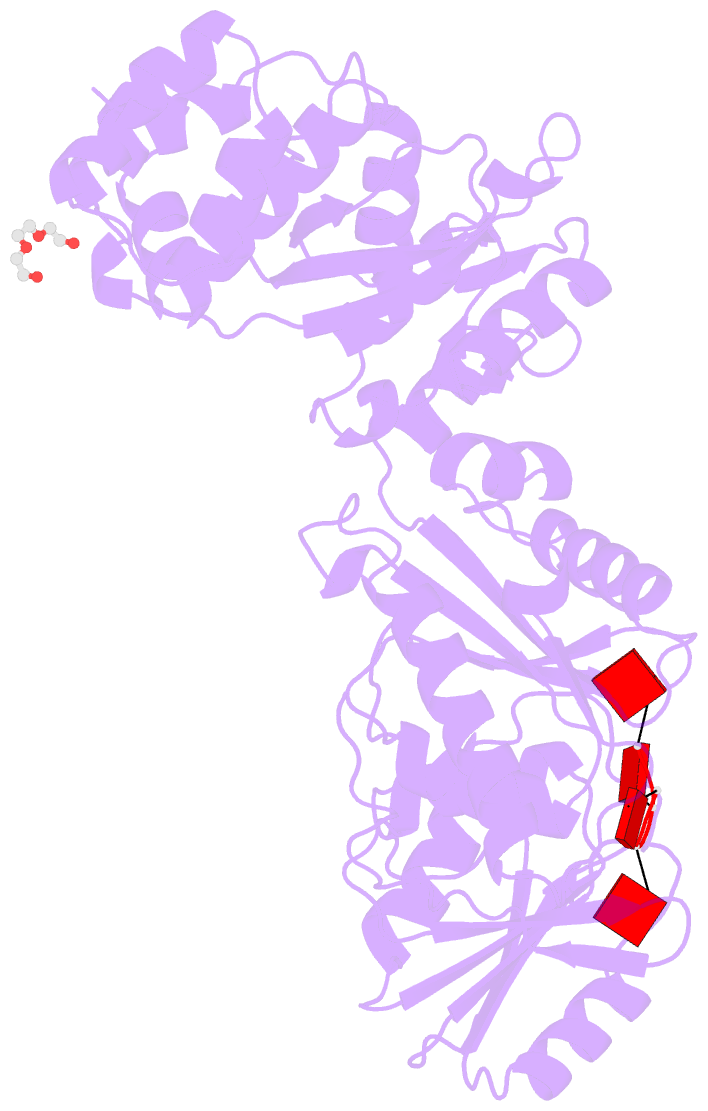
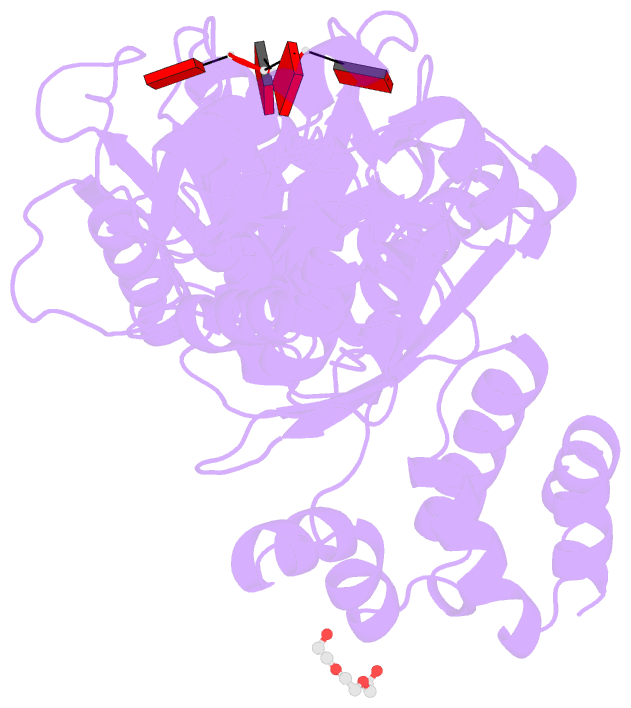
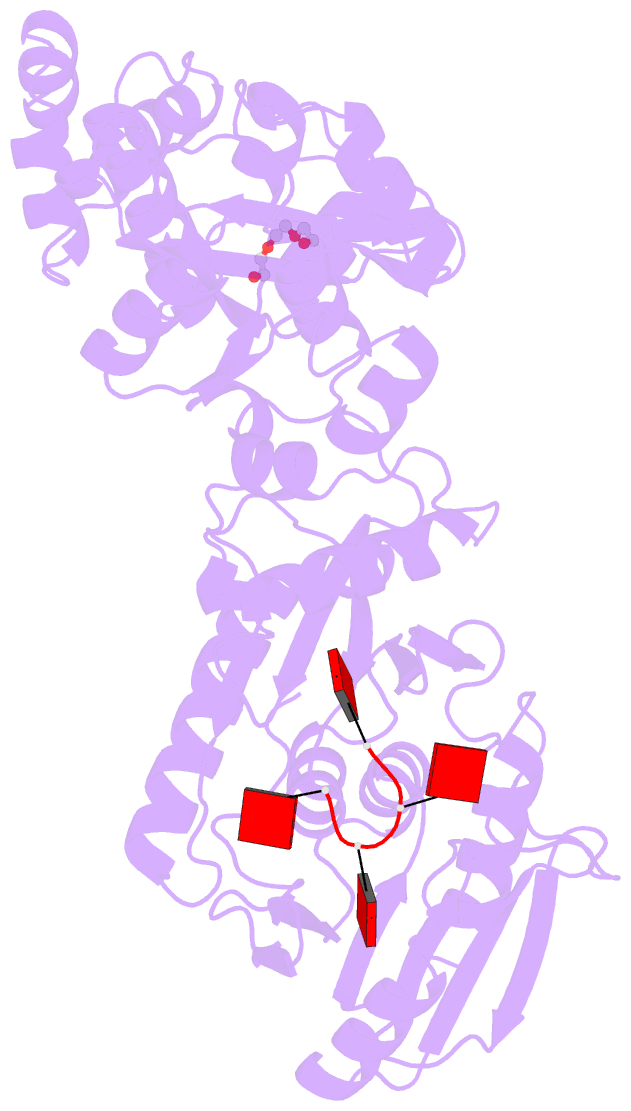
- Structure of the calpl-ca4 complex
- Reference
- Rouillon C, Schneberger N, Chi H, Blumenstock K, Da Vela S, Ackermann K, Moecking J, Peter MF, Boenigk W, Seifert R, Bode BE, Schmid-Burgk JL, Svergun D, Geyer M, White MF, Hagelueken G (2023): "Antiviral signalling by a cyclic nucleotide activated CRISPR protease." Nature, 614, 168-174. doi: 10.1038/s41586-022-05571-7.
- Abstract
- CRISPR defense systems such as the well-known DNA-targeting Cas9 and the RNA-targeting type III systems are widespread in prokaryotes1,2. The latter can orchestrate a complex antiviral response that is initiated by the synthesis of cyclic oligoadenylates (cOAs) upon foreign RNA recognition3-5. Among a large set of proteins that were linked to type III systems and predicted to bind cOAs6,7, a CRISPR associated Lon protease (CalpL) stood out to us. The protein contains a sensor domain of the SAVED (SMODS-associated and fused to various effector domains) family7, fused to a Lon protease effector domain. However, the mode of action of this effector was unknown. Here, we report the structure and function of CalpL and show that the soluble protein forms a stable tripartite complex with two further proteins, CalpT and CalpS, that are encoded in the same operon. Upon activation by cA4, CalpL oligomerizes and specifically cleaves the MazF-homolog CalpT, releasing the extracytoplasmic function (ECF) sigma factor CalpS from the complex. This provides a direct connection between CRISPR-based foreign nucleic acid detection and transcriptional regulation. Furthermore, the presence of a cA4-binding SAVED domain in a CRISPR effector reveals an unexpected link to the cyclic oligonucleotide-based antiphage signaling system (CBASS).