Summary information and primary citation
- PDB-id
- 8b4h; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- cryo-EM (3.35 Å)
- Summary
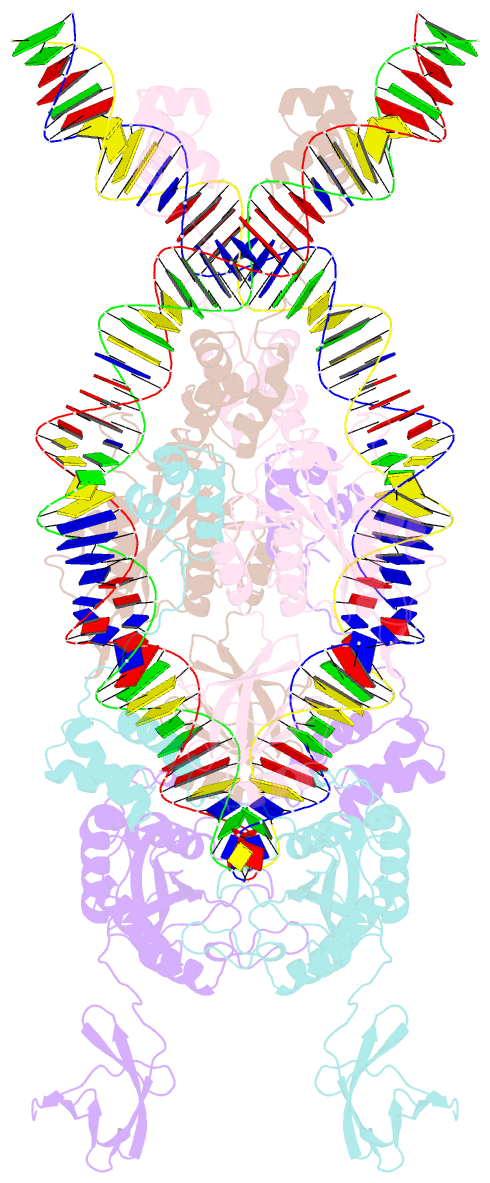
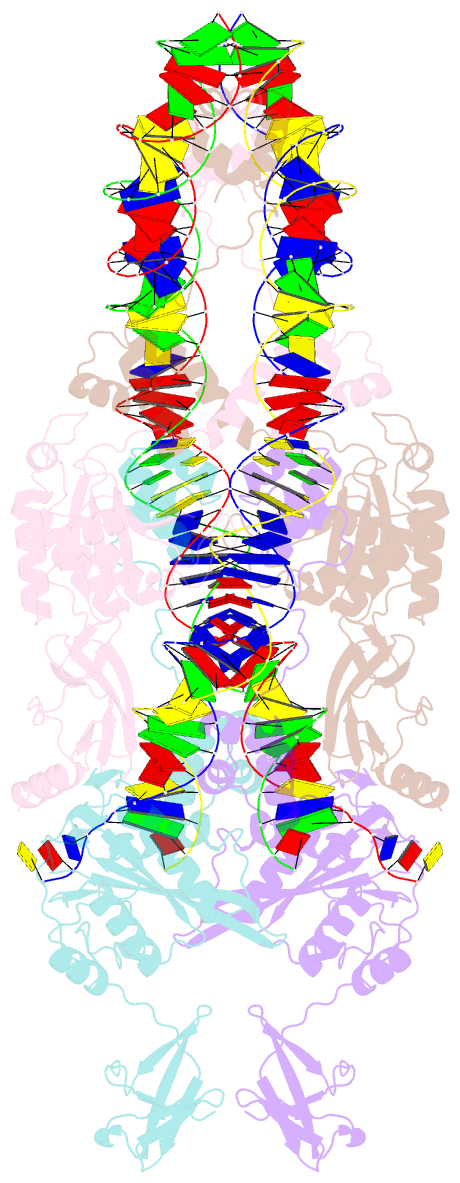
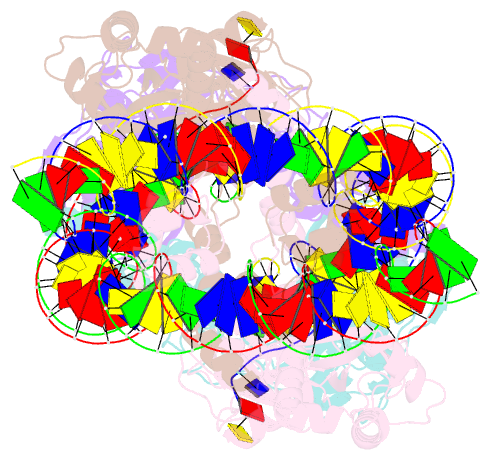
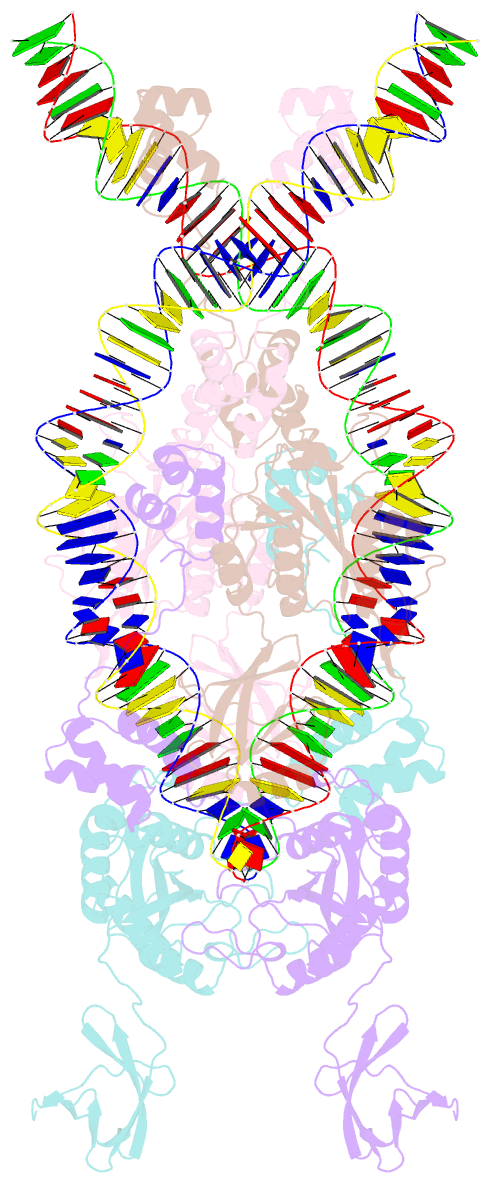
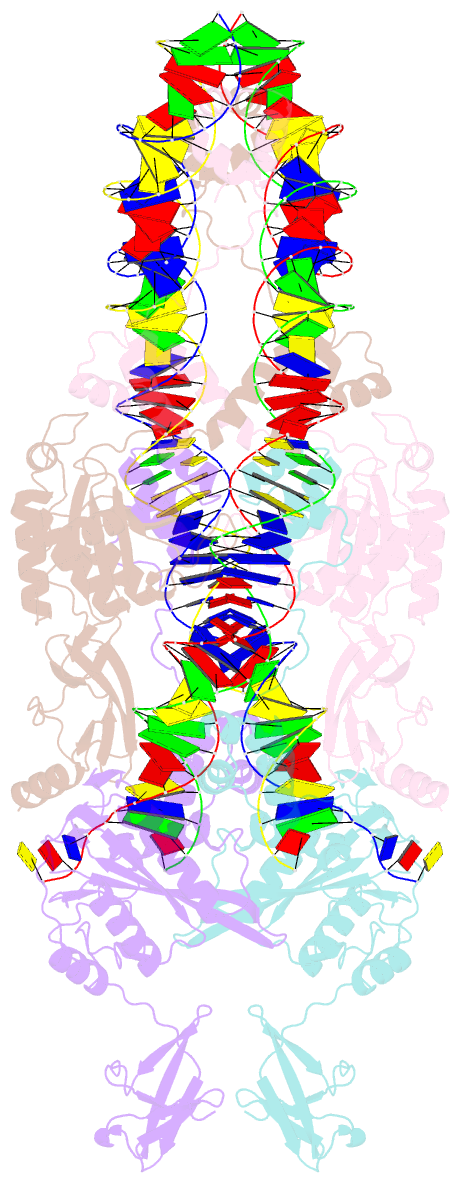
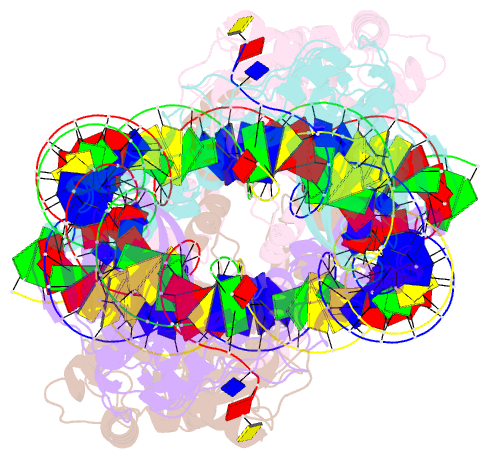
- Ista transposase cleaved donor complex
- Reference
- Spinola-Amilibia M, Araujo-Bazan L, de la Gandara A, Berger JM, Arias-Palomo E (2023): "IS21 family transposase cleaved donor complex traps two right-handed superhelical crossings." Nat Commun, 14, 2335. doi: 10.1038/s41467-023-38071-x.
- Abstract
- Transposases are ubiquitous enzymes that catalyze DNA rearrangement events with broad impacts on gene expression, genome evolution, and the spread of drug-resistance in bacteria. Here, we use biochemical and structural approaches to define the molecular determinants by which IstA, a transposase present in the widespread IS21 family of mobile elements, catalyzes efficient DNA transposition. Solution studies show that IstA engages the transposon terminal sequences to form a high-molecular weight complex and promote DNA integration. A 3.4 Å resolution structure of the transposase bound to transposon ends corroborates our biochemical findings and reveals that IstA self-assembles into a highly intertwined tetramer that synapses two supercoiled terminal inverted repeats. The three-dimensional organization of the IstA•DNA cleaved donor complex reveals remarkable similarities with retroviral integrases and classic transposase systems, such as Tn7 and bacteriophage Mu, and provides insights into IS21 transposition.