Summary information and primary citation
- PDB-id
- 8bw5; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase
- Method
- X-ray (2.8 Å)
- Summary
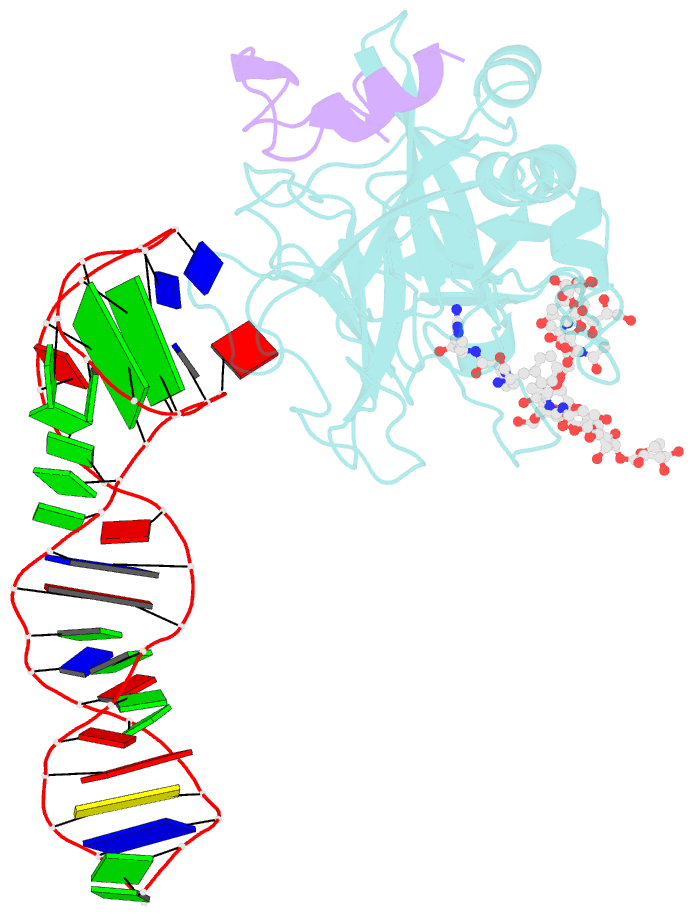
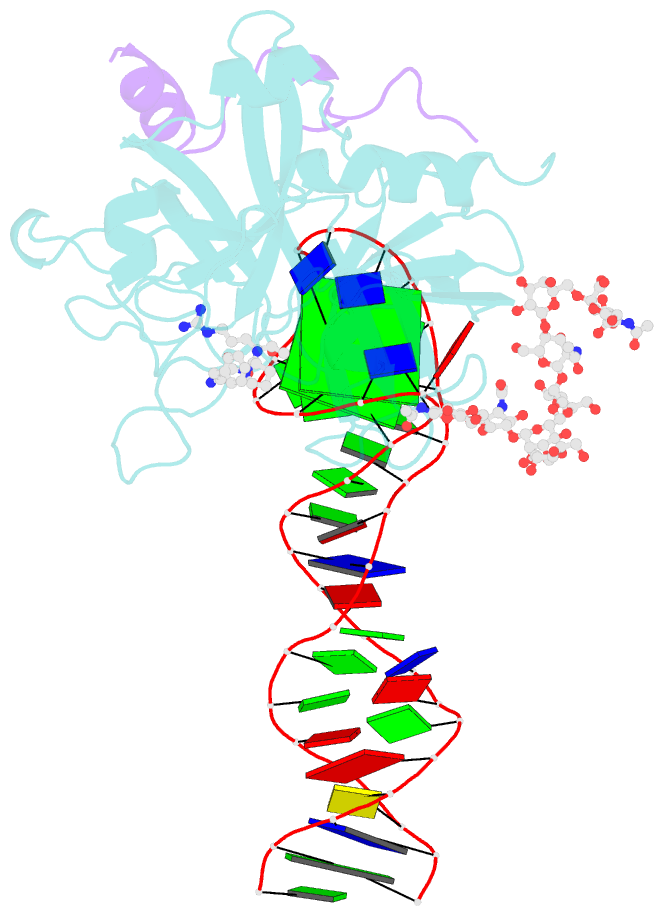
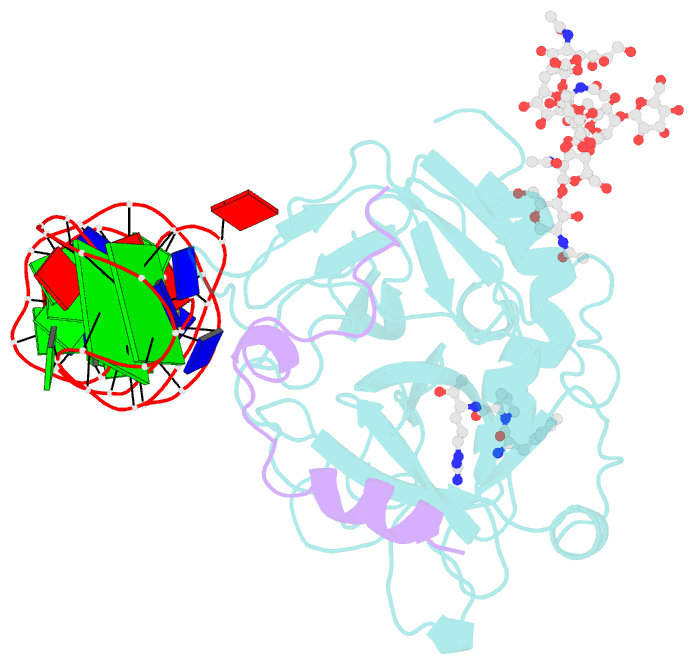
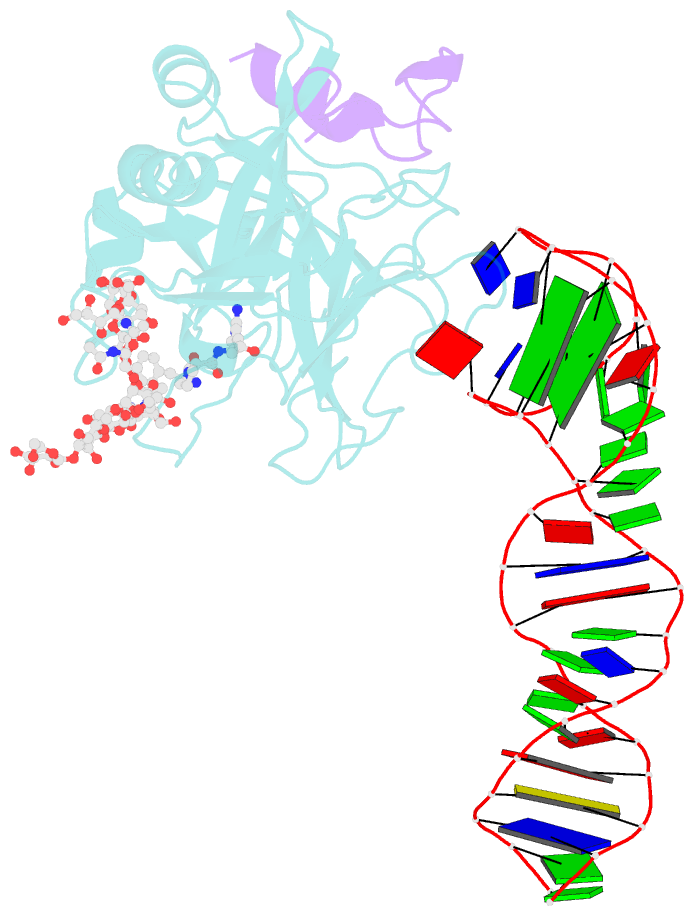
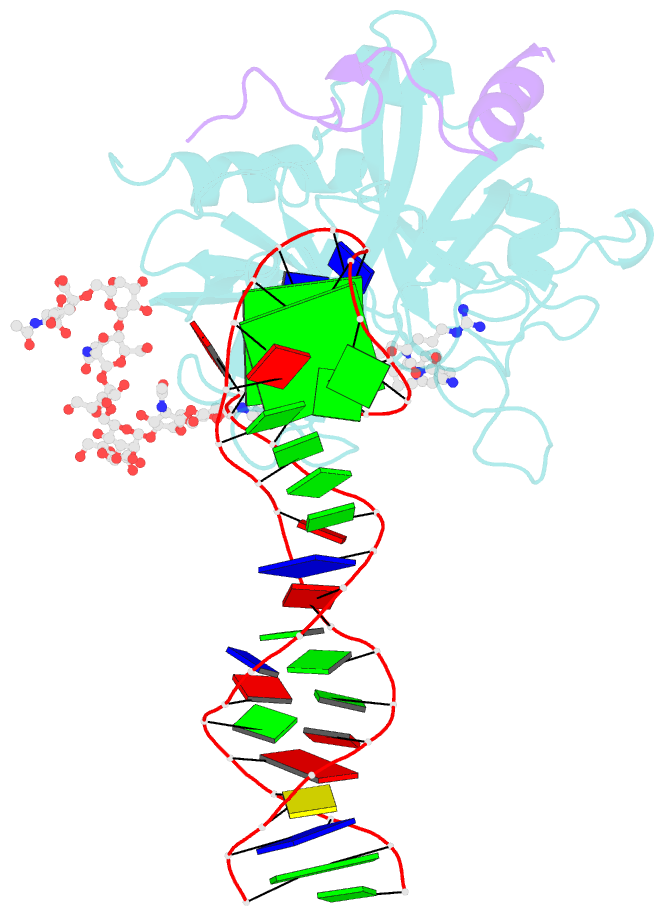
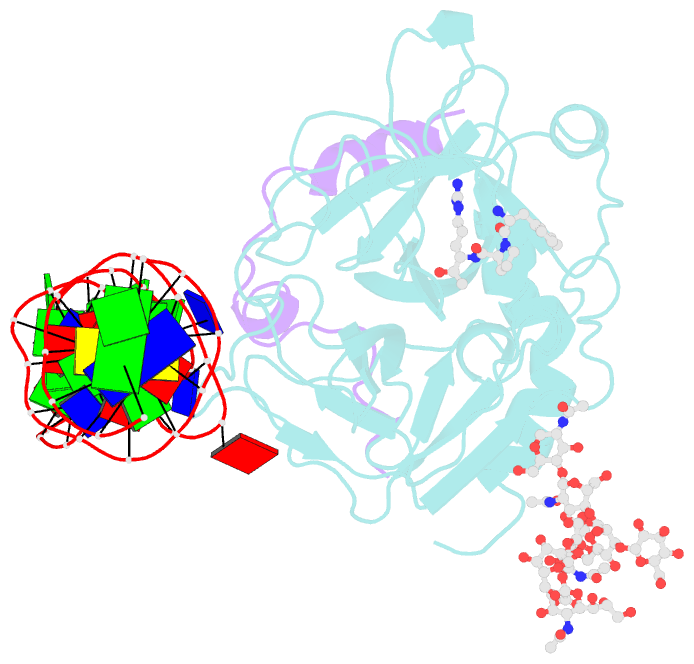
- X-ray structure of the complex between human alpha thrombin and the duplex-quadruplex aptamer m08s-1_41mer
- Reference
- Troisi R, Napolitano V, Rossitto E, Osman W, Nagano M, Wakui K, Popowicz GM, Yoshimoto K, Sica F (2023): "Steric hindrance and structural flexibility shape the functional properties of a guanine-rich oligonucleotide." Nucleic Acids Res., 51, 8880-8890. doi: 10.1093/nar/gkad634.
- Abstract
- Ligand/protein molecular recognition involves a dynamic process, whereby both partners require a degree of structural plasticity to regulate the binding/unbinding event. Here, we present the characterization of the interaction between a highly dynamic G-rich oligonucleotide, M08s-1, and its target protein, human α-thrombin. M08s-1 is the most active anticoagulant aptamer selected thus far. Circular dichroism and gel electrophoresis analyses indicate that both intramolecular and intermolecular G-quadruplex structures are populated in solution. The presence of thrombin stabilises the antiparallel intramolecular chair-like G-quadruplex conformation, that provides by far the main contribution to the biological activity of the aptamer. The crystal structure of the thrombin-oligonucleotide complex reveals that M08s-1 adopts a kinked structural organization formed by a G-quadruplex domain and a long duplex module, linked by a stretch of five purine bases. The quadruplex motif hooks the exosite I region of thrombin and the duplex region is folded towards the surface of the protein. This structural feature, which has never been observed in other anti-exosite I aptamers with a shorter duplex motif, hinders the approach of a protein substrate to the active site region and may well explain the significant increase in the anticoagulant activity of M08s-1 compared to the other anti-exosite I aptamers.