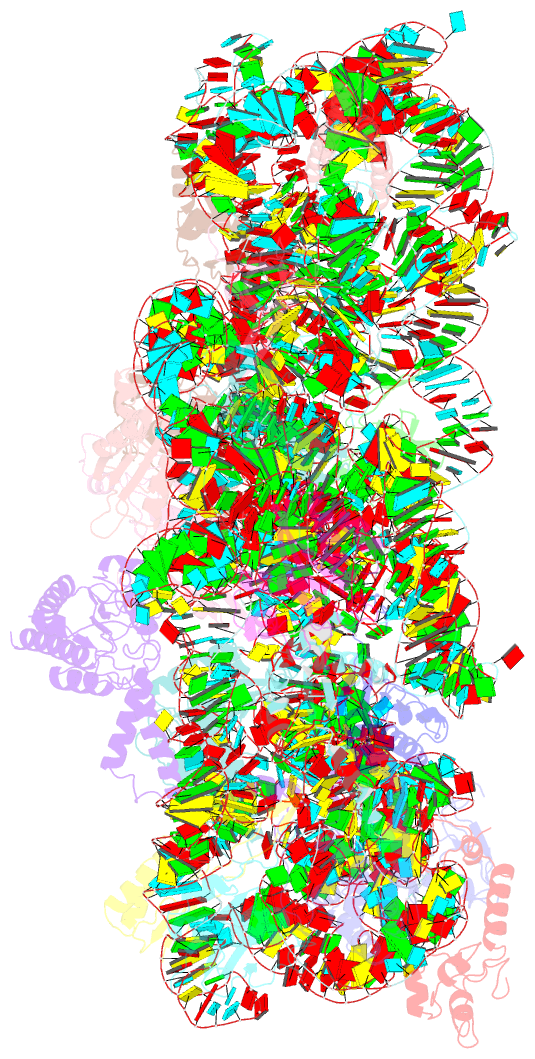
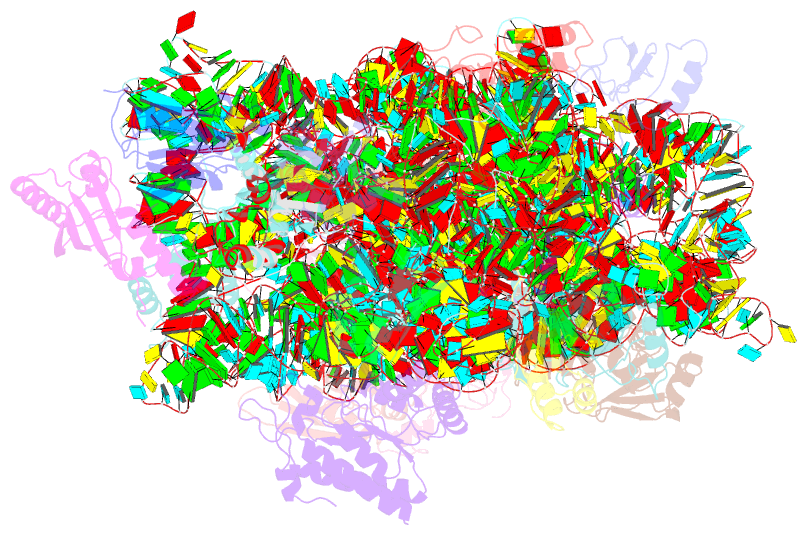
Summary information and primary citation
- PDB-id
- 8byv; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- cryo-EM (2.89 Å)
- Summary
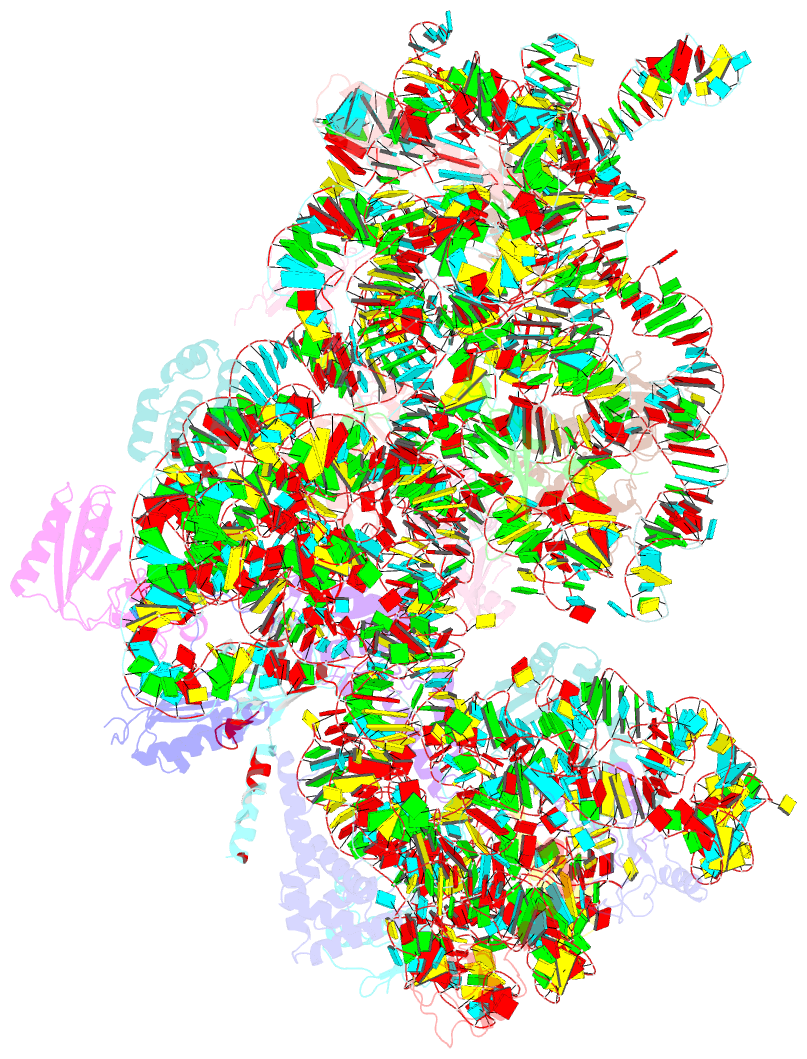
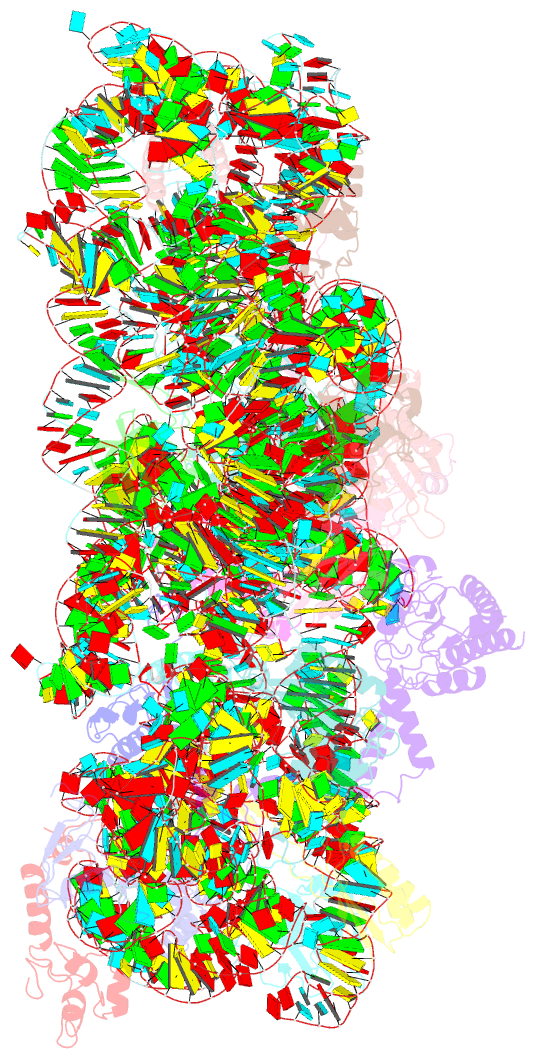
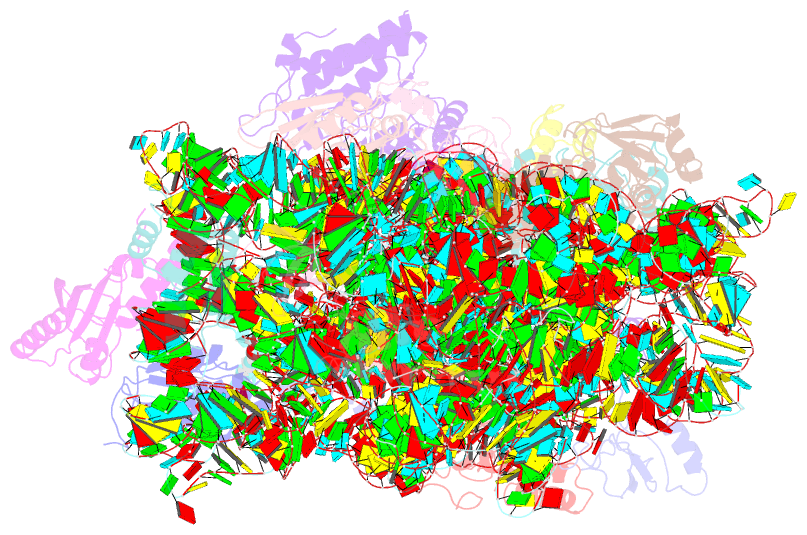
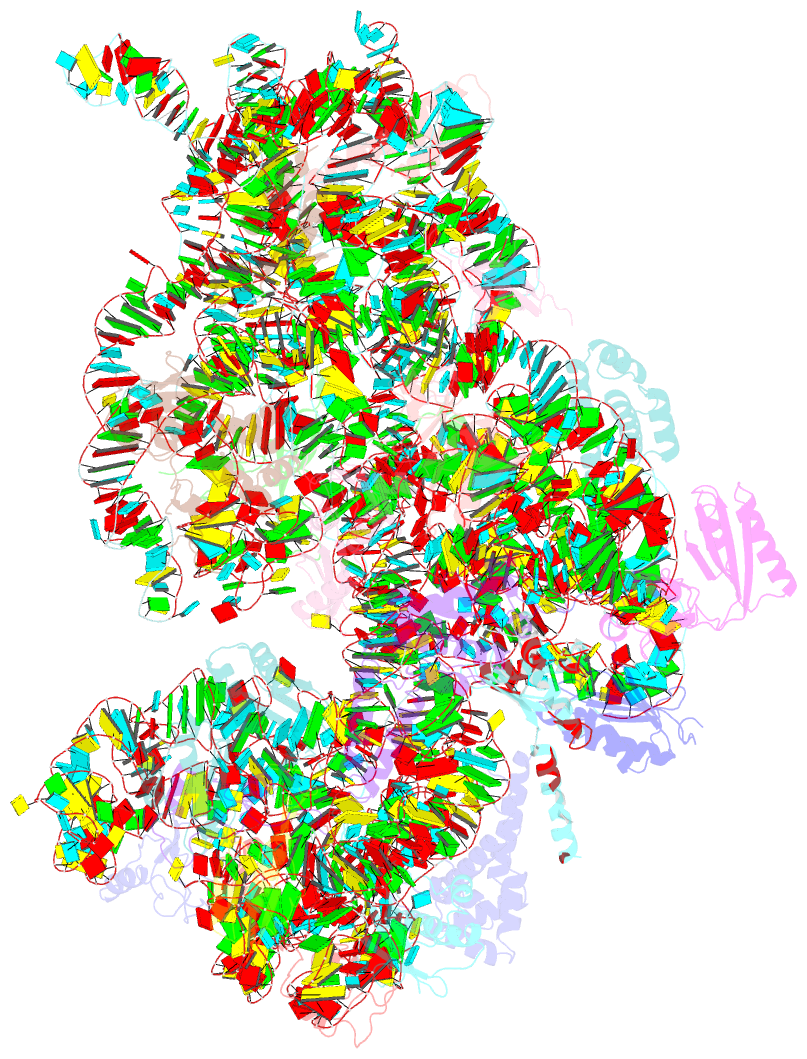
- cryo-EM structure of a staphylococus aureus 30s-rbfa complex
- Reference
- Bikmullin AG, Fatkhullin B, Stetsenko A, Gabdulkhakov A, Garaeva N, Nurullina L, Klochkova E, Golubev A, Khusainov I, Trachtmann N, Blokhin D, Guskov A, Validov S, Usachev K, Yusupov M (2023): "Yet Another Similarity between Mitochondrial and Bacterial Ribosomal Small Subunit Biogenesis Obtained by Structural Characterization of RbfA from S. aureus." Int J Mol Sci, 24. doi: 10.3390/ijms24032118.
- Abstract
- Ribosome biogenesis is a complex and highly accurate conservative process of ribosomal subunit maturation followed by association. Subunit maturation comprises sequential stages of ribosomal RNA and proteins' folding, modification and binding, with the involvement of numerous RNAses, helicases, GTPases, chaperones, RNA, protein-modifying enzymes, and assembly factors. One such assembly factor involved in bacterial 30S subunit maturation is ribosomal binding factor A (RbfA). In this study, we present the crystal (determined at 2.2 Å resolution) and NMR structures of RbfA as well as the 2.9 Å resolution cryo-EM reconstruction of the 30S-RbfA complex from Staphylococcus aureus (S. aureus). Additionally, we show that the manner of RbfA action on the small ribosomal subunit during its maturation is shared between bacteria and mitochondria. The obtained results clarify the function of RbfA in the 30S maturation process and its role in ribosome functioning in general. Furthermore, given that S. aureus is a serious human pathogen, this study provides an additional prospect to develop antimicrobials targeting bacterial pathogens.