Summary information and primary citation
- PDB-id
- 8cti; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-RNA
- Method
- cryo-EM (3.6 Å)
- Summary
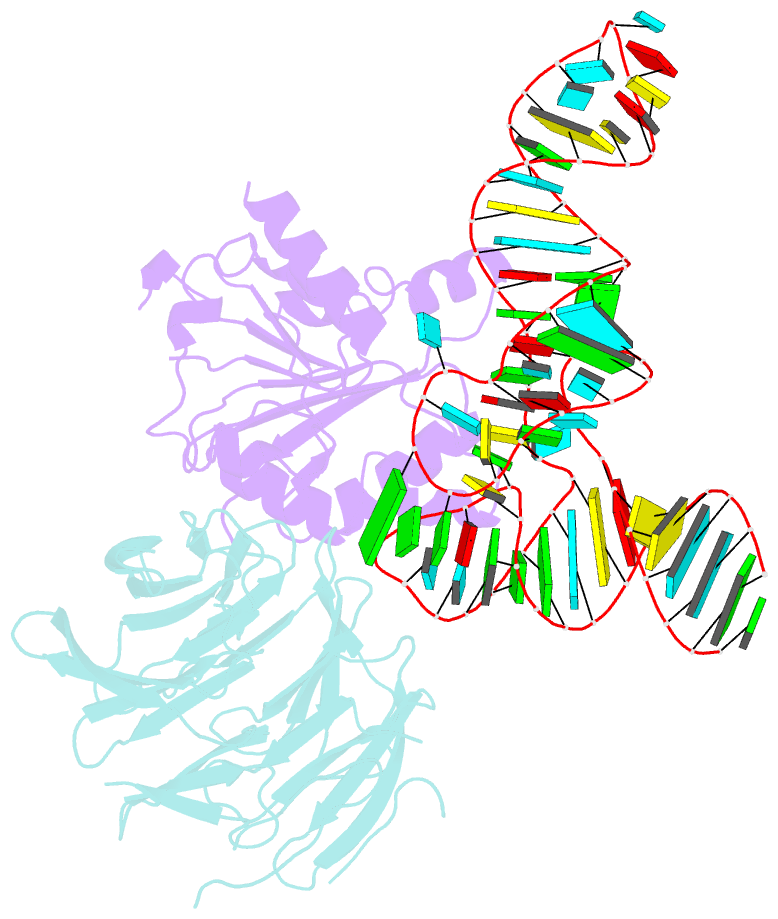
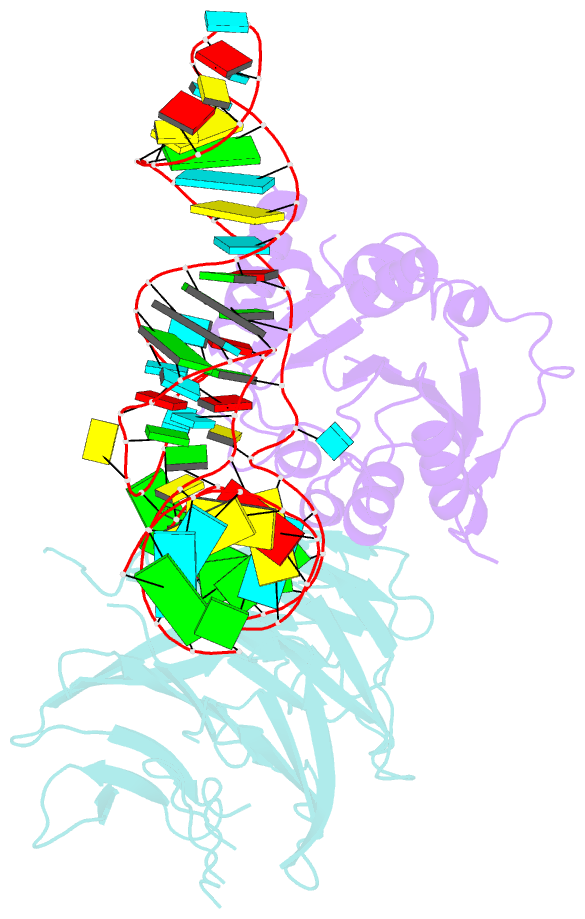
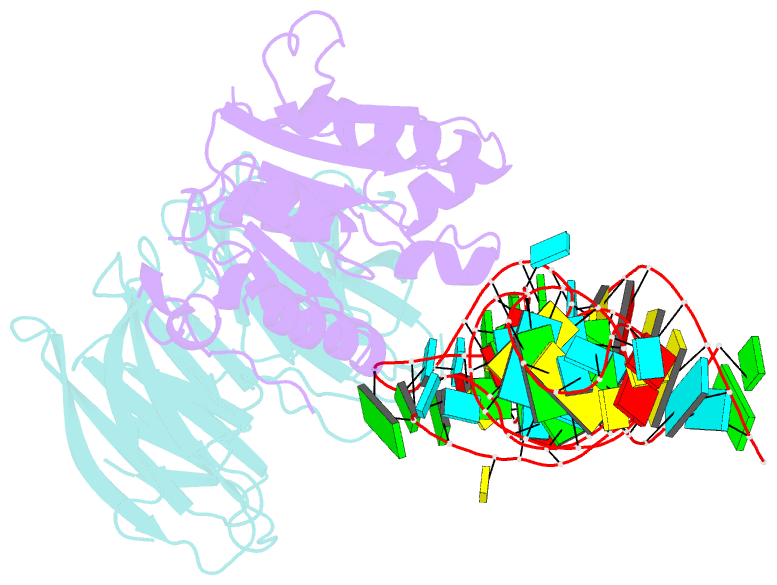
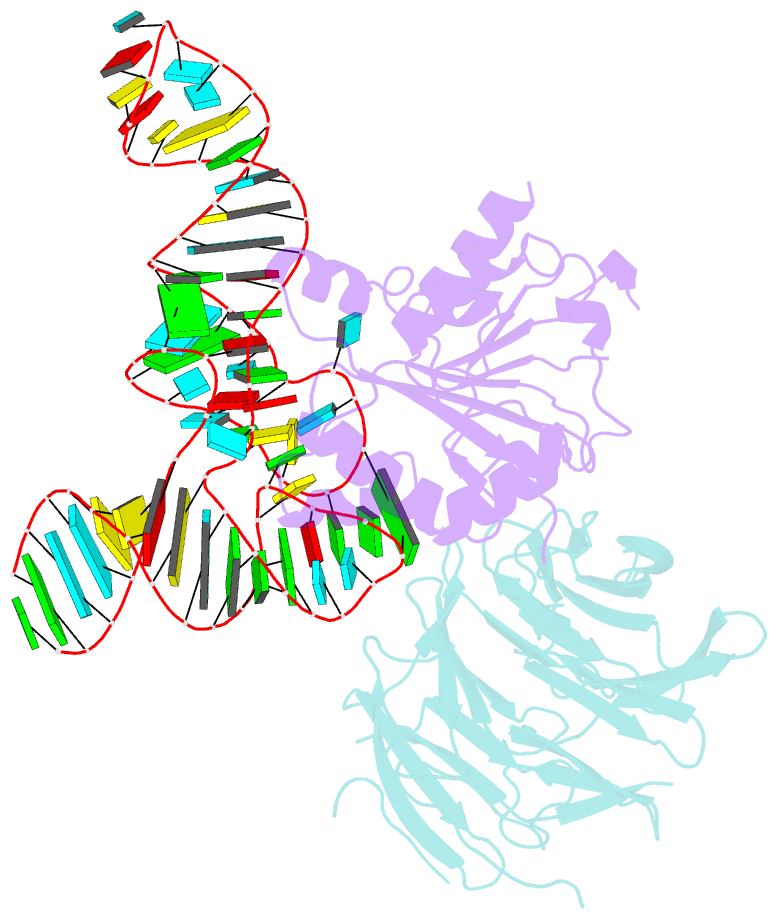
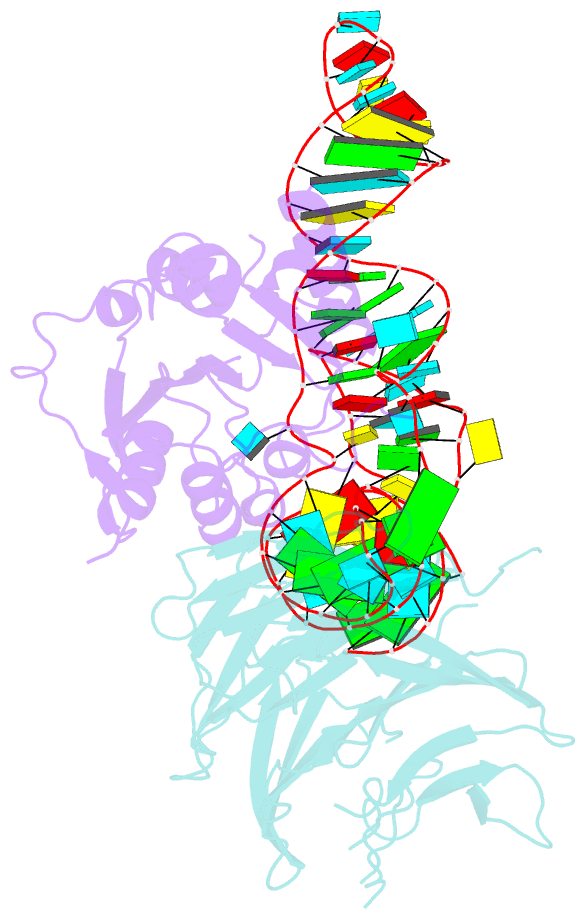
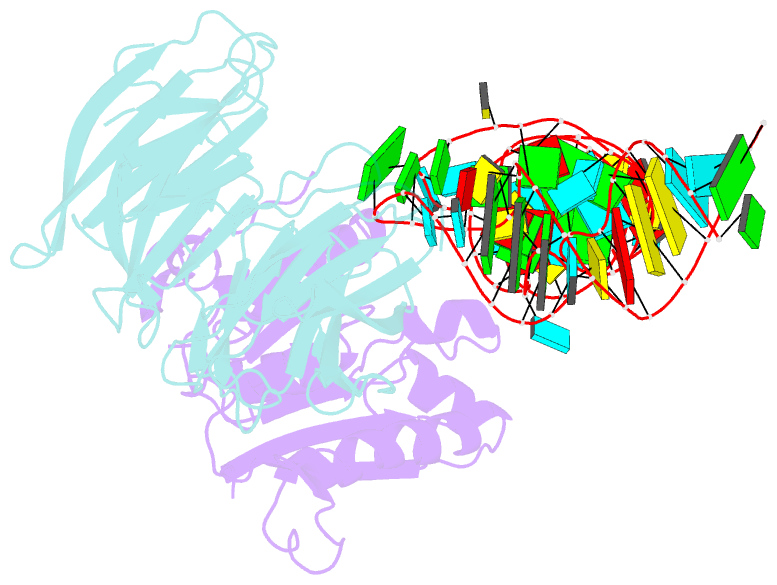
- cryo-EM structure of human mettl1-wdr4-trna(val) complex
- Reference
- Li J, Wang L, Hahn Q, Nowak RP, Viennet T, Orellana EA, Roy Burman SS, Yue H, Hunkeler M, Fontana P, Wu H, Arthanari H, Fischer ES, Gregory RI (2023): "Structural basis of regulated m 7 G tRNA modification by METTL1-WDR4." Nature, 613, 391-397. doi: 10.1038/s41586-022-05566-4.
- Abstract
- Chemical modifications of RNA have key roles in many biological processes1-3. N7-methylguanosine (m7G) is required for integrity and stability of a large subset of tRNAs4-7. The methyltransferase 1-WD repeat-containing protein 4 (METTL1-WDR4) complex is the methyltransferase that modifies G46 in the variable loop of certain tRNAs, and its dysregulation drives tumorigenesis in numerous cancer types8-14. Mutations in WDR4 cause human developmental phenotypes including microcephaly15-17. How METTL1-WDR4 modifies tRNA substrates and is regulated remains elusive18. Here we show, through structural, biochemical and cellular studies of human METTL1-WDR4, that WDR4 serves as a scaffold for METTL1 and the tRNA T-arm. Upon tRNA binding, the αC region of METTL1 transforms into a helix, which together with the α6 helix secures both ends of the tRNA variable loop. Unexpectedly, we find that the predicted disordered N-terminal region of METTL1 is part of the catalytic pocket and essential for methyltransferase activity. Furthermore, we reveal that S27 phosphorylation in the METTL1 N-terminal region inhibits methyltransferase activity by locally disrupting the catalytic centre. Our results provide a molecular understanding of tRNA substrate recognition and phosphorylation-mediated regulation of METTL1-WDR4, and reveal the presumed disordered N-terminal region of METTL1 as a nexus of methyltransferase activity.