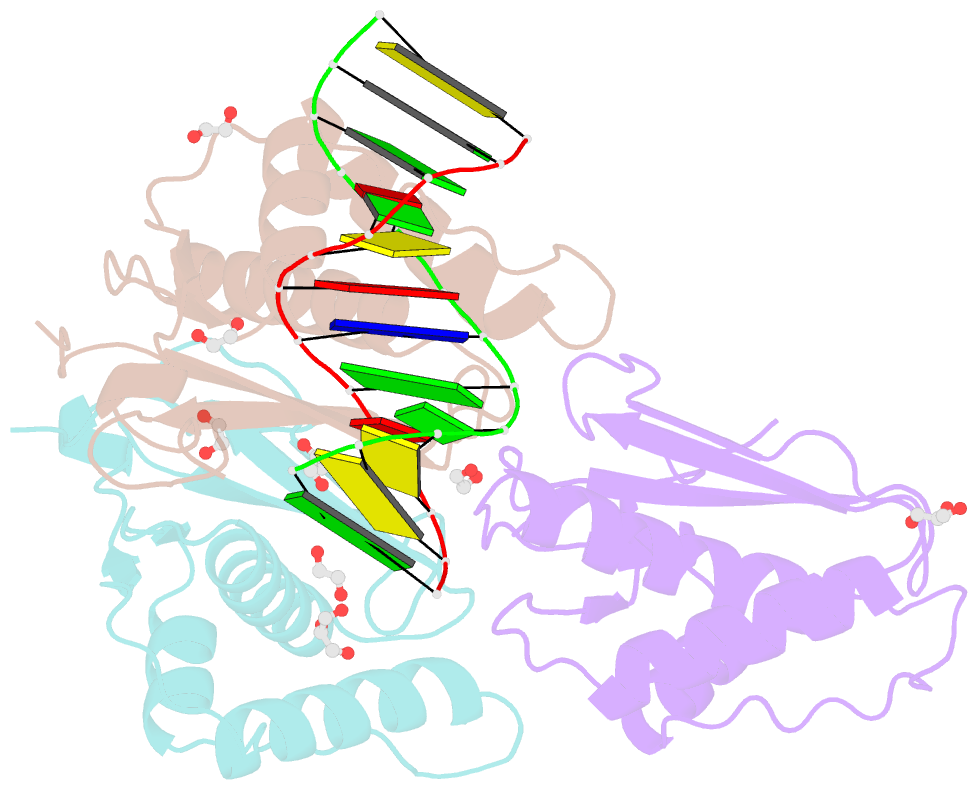
Summary information and primary citation
- PDB-id
- 8cu0; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA
- Method
- X-ray (1.74 Å)
- Summary
- 12-mer DNA structure of exbim bound to rnaseh -modified ddd
- Reference
- Kellum Jr AH, Pallan PS, Nilforoushan A, Sturla SJ, Stone MP, Egli M (2022): "Conformation and Pairing Properties of an O 6 -Methyl-2'-deoxyguanosine-Directed Benzimidazole Nucleoside Analog in Duplex DNA." Chem.Res.Toxicol., 35, 1903-1913. doi: 10.1021/acs.chemrestox.2c00165.
- Abstract
- O6-Methyl-2'-deoxyguanosine (O6-MeG) is one of the most common DNA lesions and arises as a consequence of both xenobiotic carcinogens and endogenous methylation by S-adenosylmethionine. O6-MeG frequently causes G-to-A mutations during DNA replication due to the misincorporation of dTTP and continued DNA synthesis. Efforts to detect DNA adducts such as O6-MeG, and to understand their impacts on DNA structure and function, have motivated the creation of nucleoside analogs with altered base moieties to afford a more favorable interaction with the adduct as compared to the unmodified nucleotide. Such analogs directed at O6-MeG include benzimidazolinone and benzimidazole nucleotides, as well as their extended π surface analogs naphthimidazolinone and napthimidazole derivatives. These analogs form a more stable pair with O6-MeG than with G, most likely due to a combination of H-bonding and stacking. While extending the π surface of the analogs enhances their performance as adduct-directed probes, the precise origins of the increased affinity between the synthetic analogs and O6-MeG remain unclear. To better understand relevant conformational and pairing properties, we used X-ray crystallography and analyzed the structures of the DNA duplexes with naphthimidazolinone inserted opposite G or O6-MeG. The structures reveal a complex interaction of the analog found either in an anti orientation and stacked inside the duplex, either above or below G or O6-MeG, or in a syn orientation and paired opposite G with formation of a single H-bond. The experimental structural data are consistent with the stabilizing effect of the synthetic analog observed in UV melting experiments and calculations and moreover reveal that the origin of these observations appears to be superior stacking between O6-MeG and the extended π system of the synthetic probe.