Summary information and primary citation
- PDB-id
- 8d4b; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-RNA
- Method
- cryo-EM (2.92 Å)
- Summary
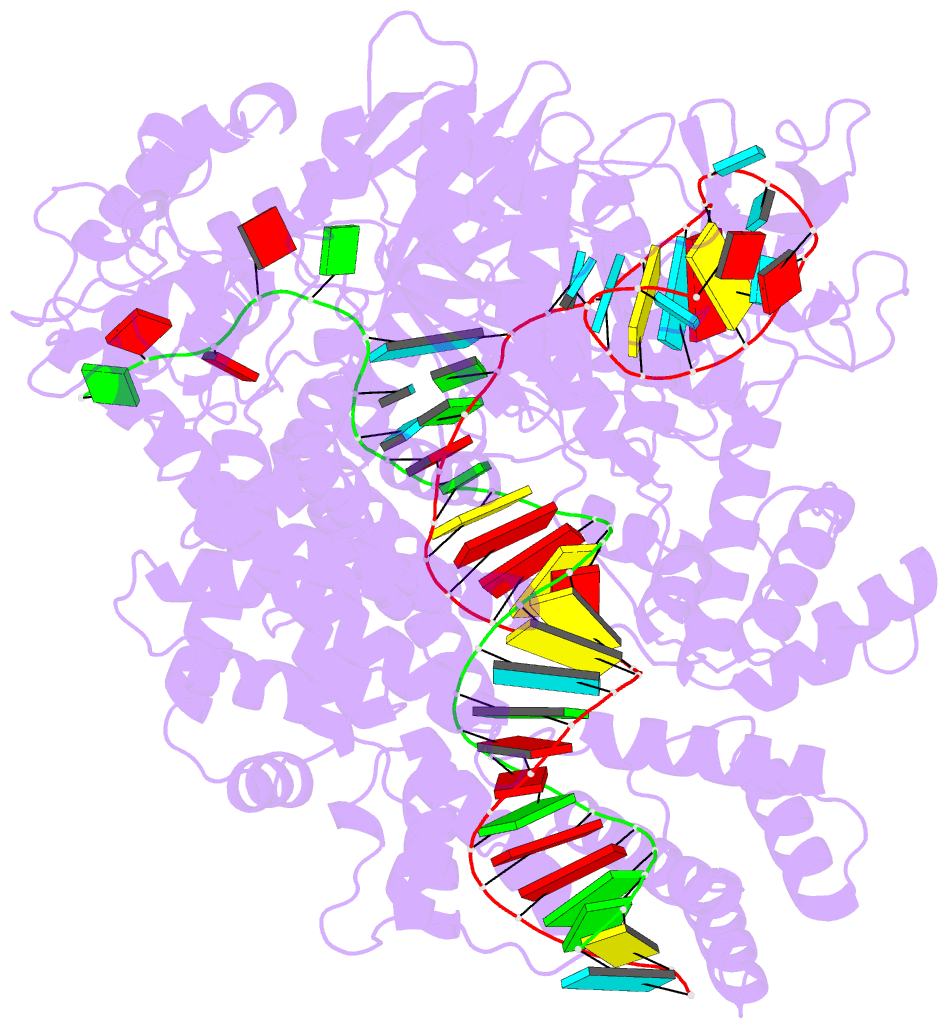
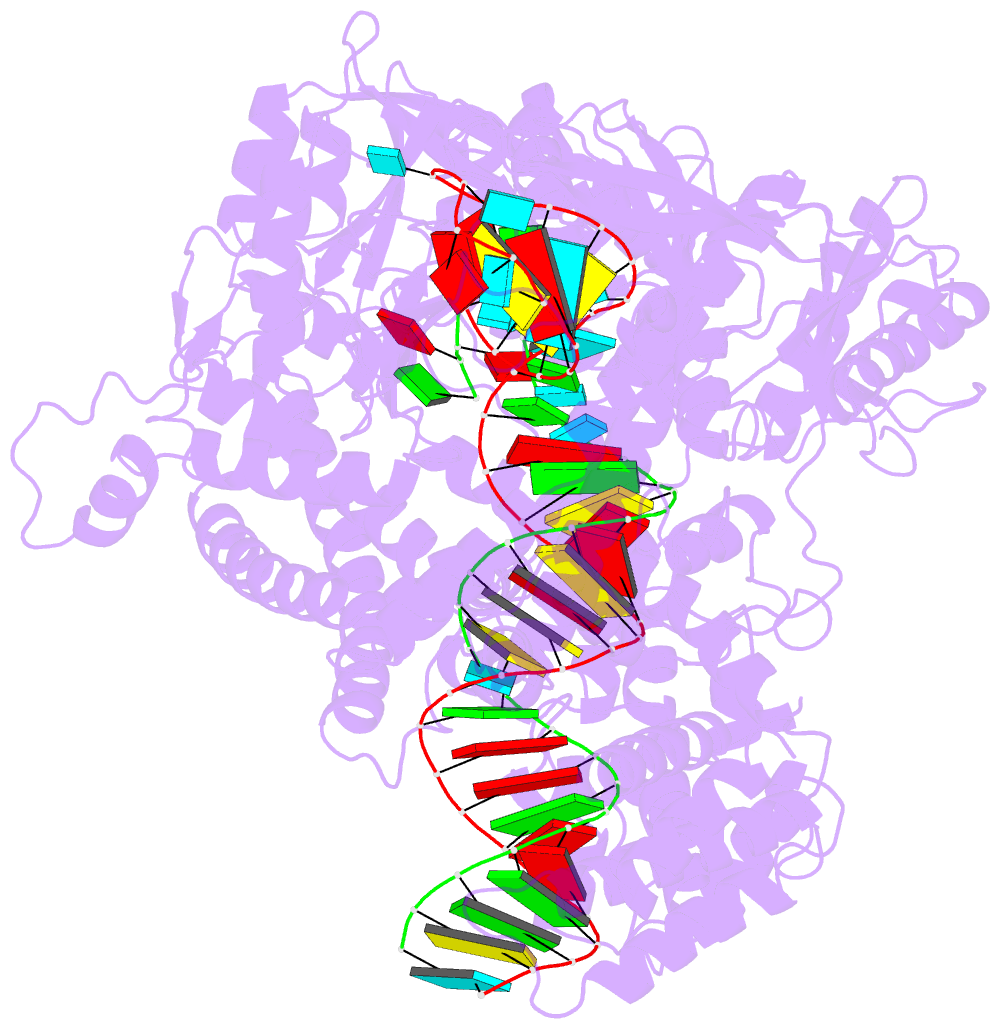
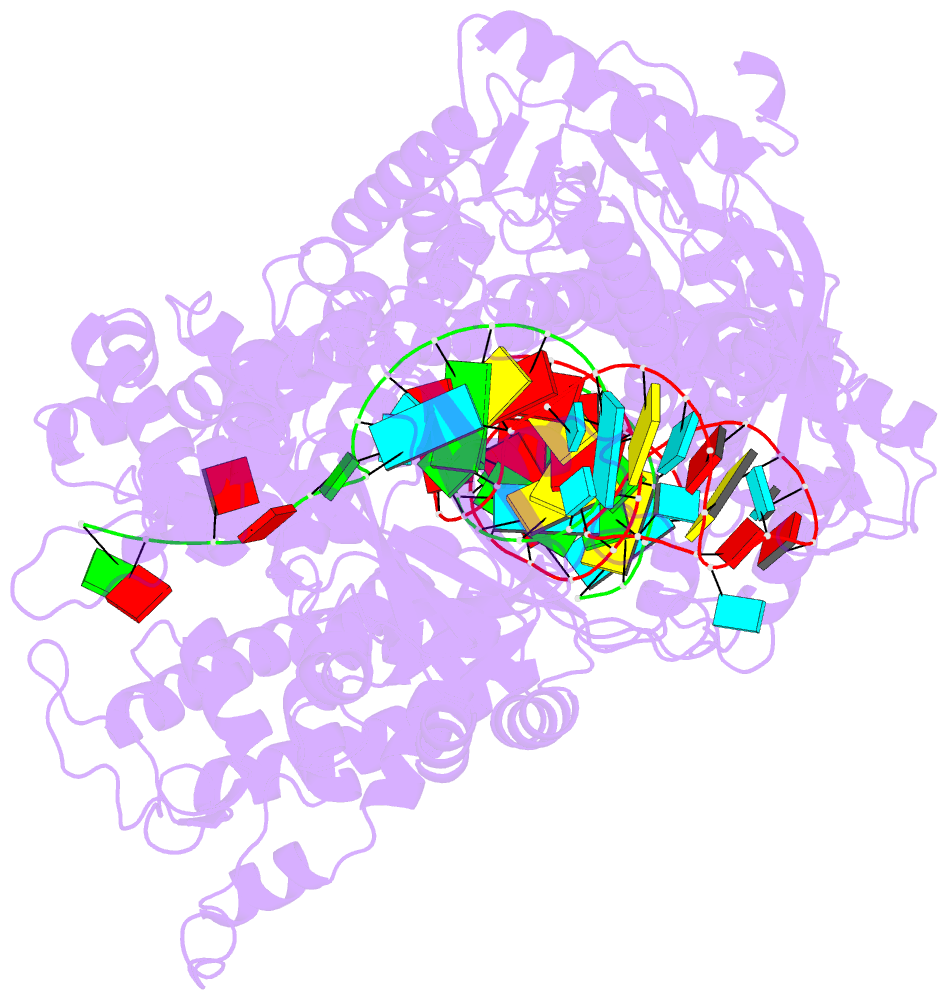
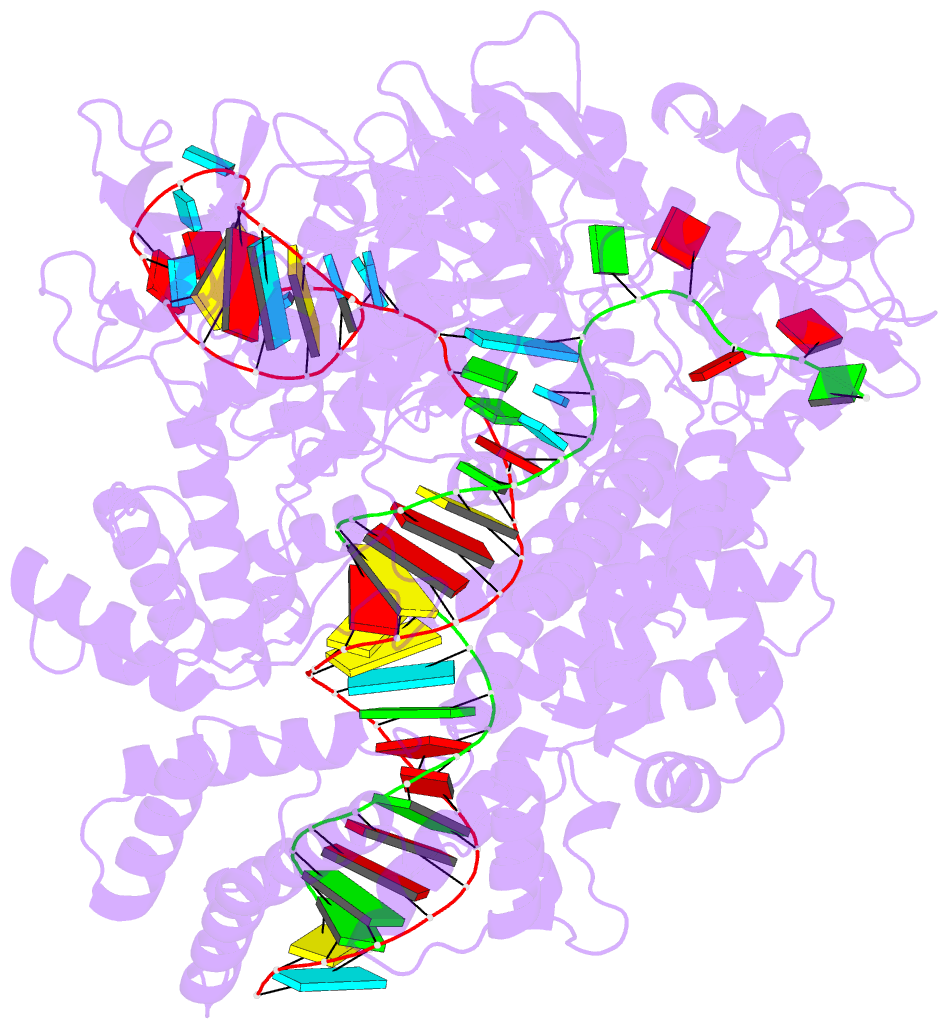
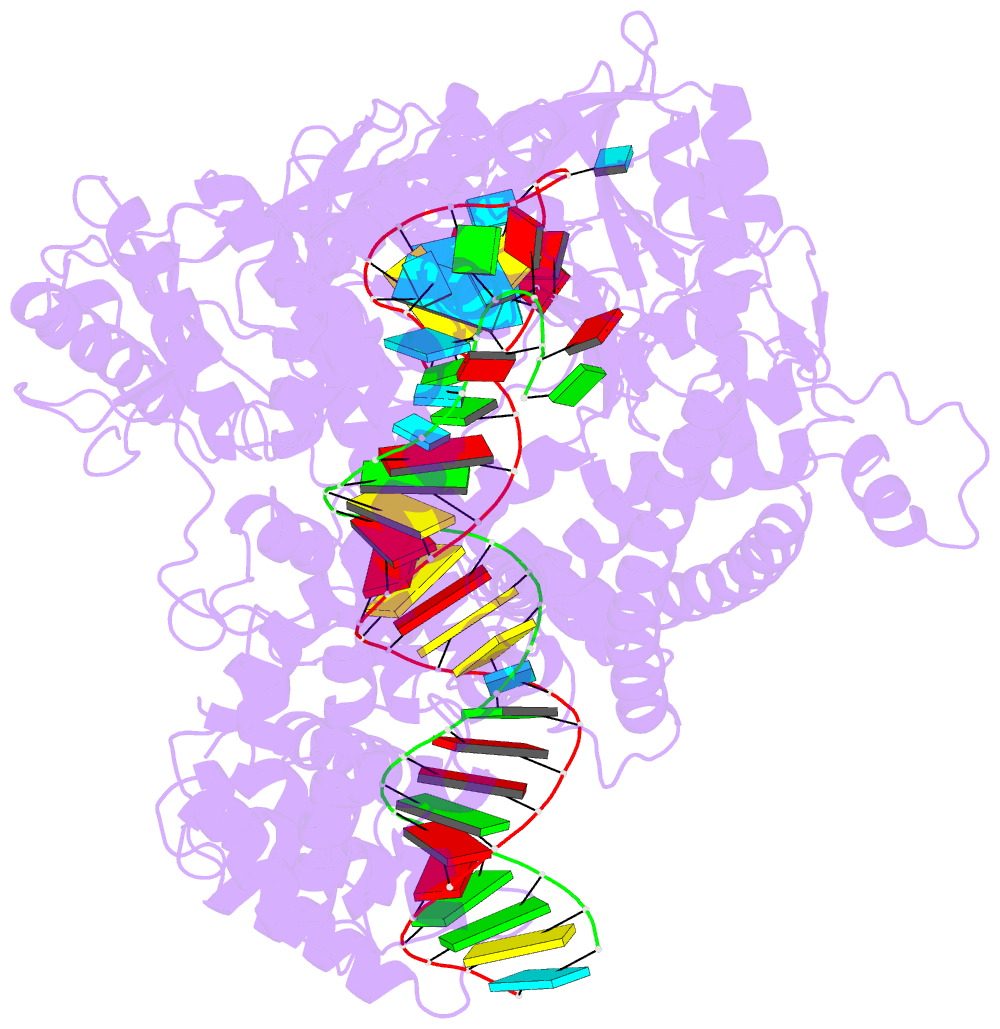
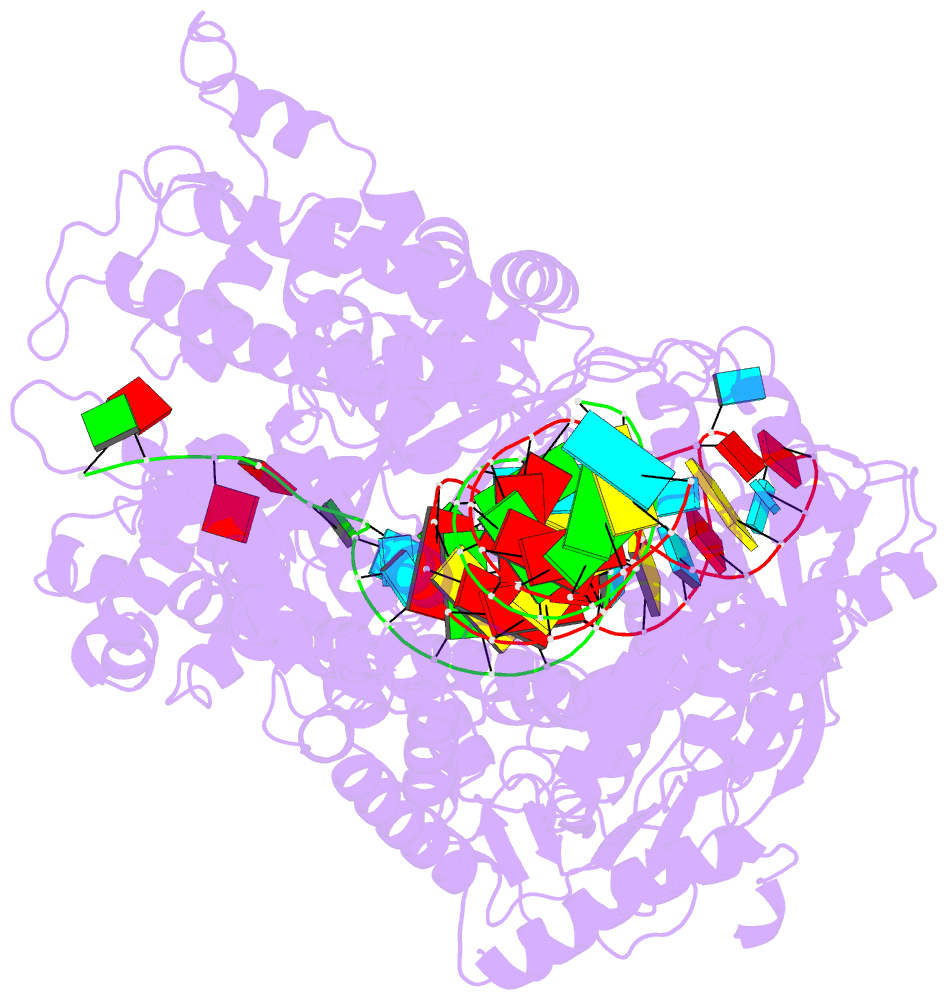
- Structure of cas12a2 ternary complex
- Reference
- Bravo JPK, Hallmark T, Naegle B, Beisel CL, Jackson RN, Taylor DW (2023): "RNA targeting unleashes indiscriminate nuclease activity of CRISPR-Cas12a2." Nature, 613, 582-587. doi: 10.1038/s41586-022-05560-w.
- Abstract
- Cas12a2 is a CRISPR-associated nuclease that performs RNA-guided, sequence-nonspecific degradation of single-stranded RNA, single-stranded DNA and double-stranded DNA following recognition of a complementary RNA target, culminating in abortive infection1. Here we report structures of Cas12a2 in binary, ternary and quaternary complexes to reveal a complete activation pathway. Our structures reveal that Cas12a2 is autoinhibited until binding a cognate RNA target, which exposes the RuvC active site within a large, positively charged cleft. Double-stranded DNA substrates are captured through duplex distortion and local melting, stabilized by pairs of 'aromatic clamp' residues that are crucial for double-stranded DNA degradation and in vivo immune system function. Our work provides a structural basis for this mechanism of abortive infection to achieve population-level immunity, which can be leveraged to create rational mutants that degrade a spectrum of collateral substrates.