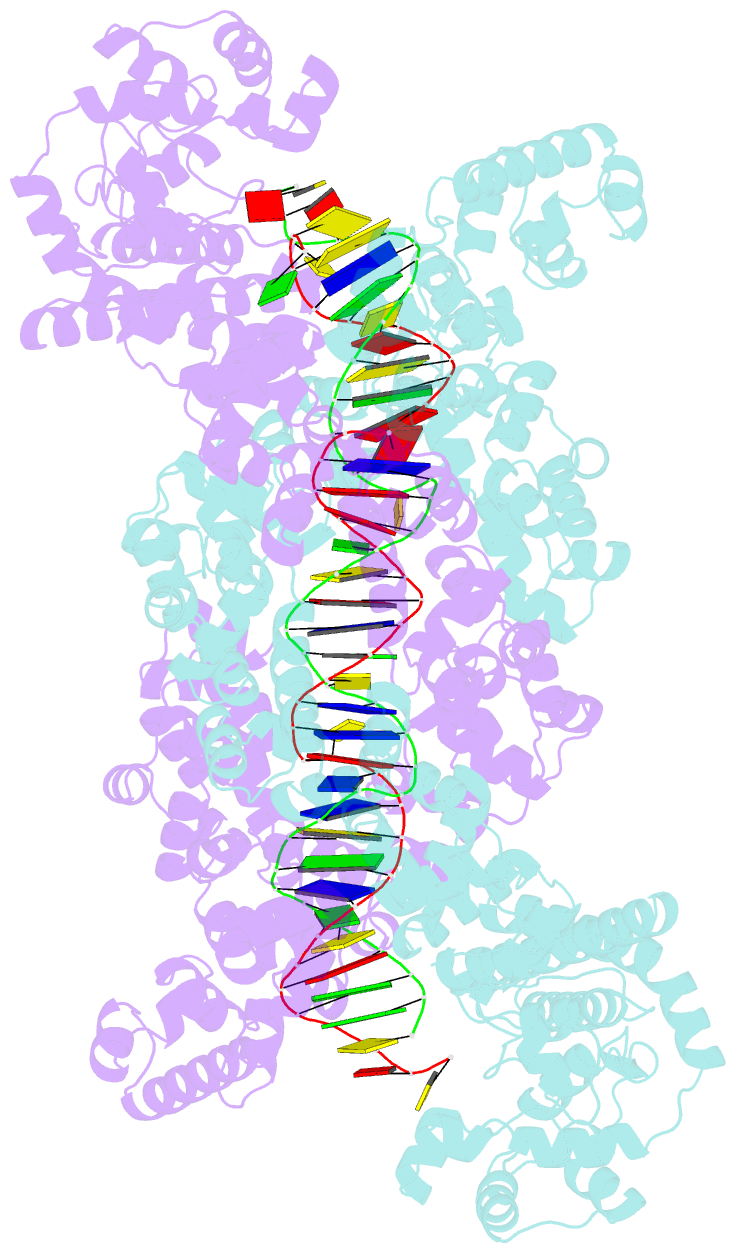
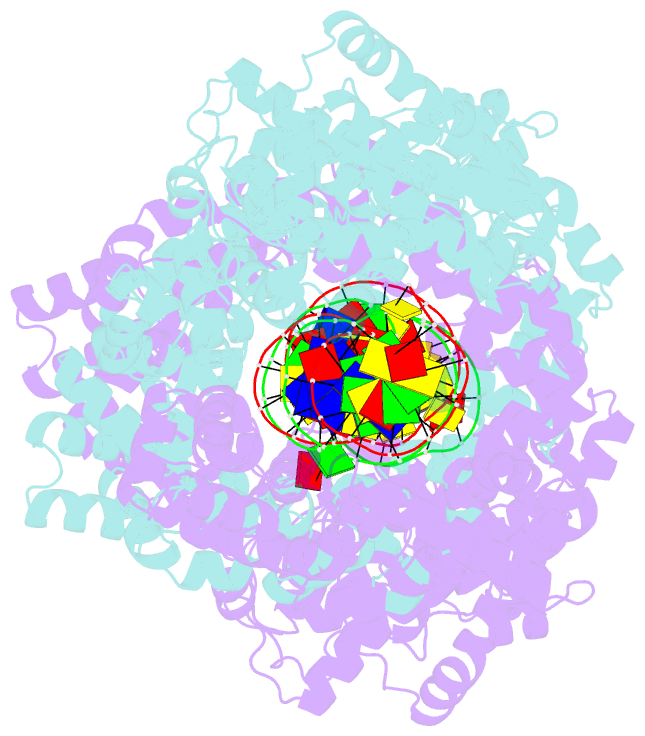
Summary information and primary citation
- PDB-id
- 8df7; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (3.52 Å)
- Summary
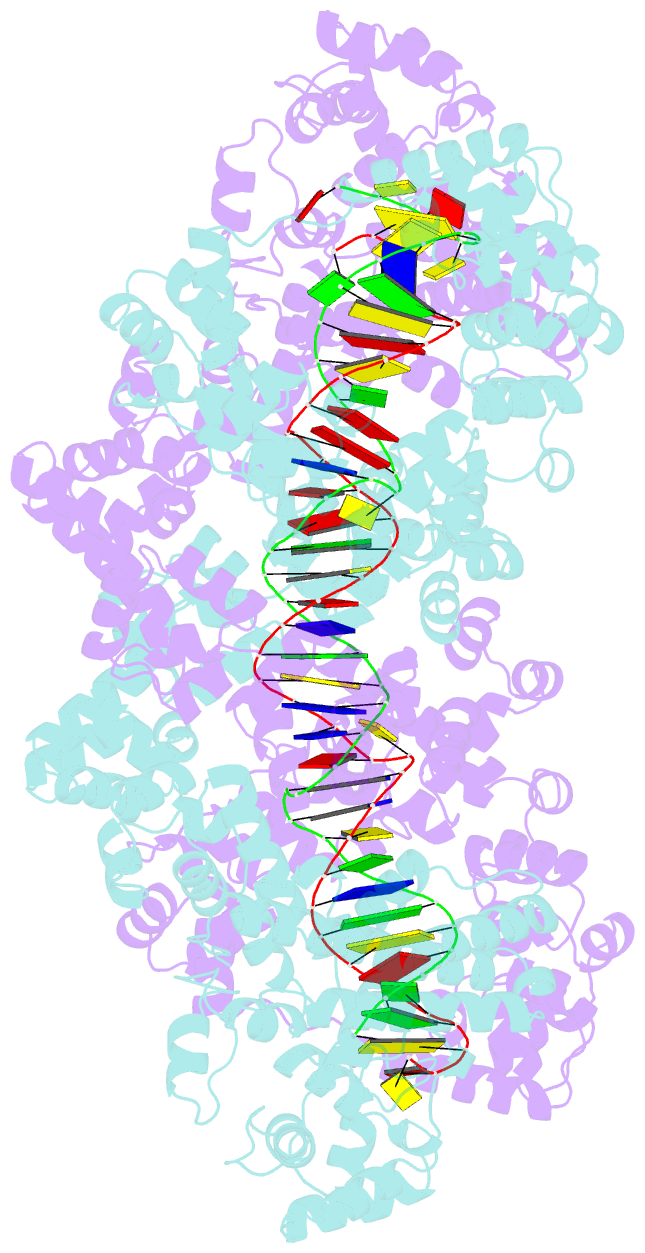
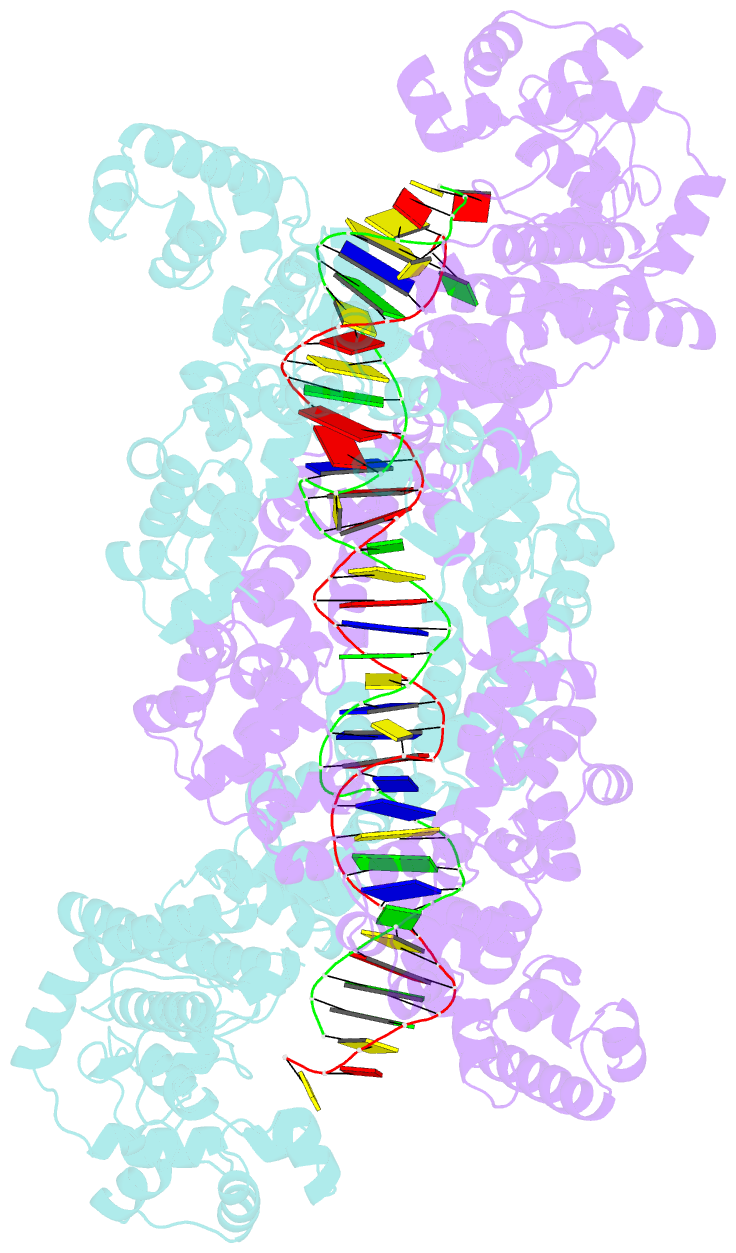
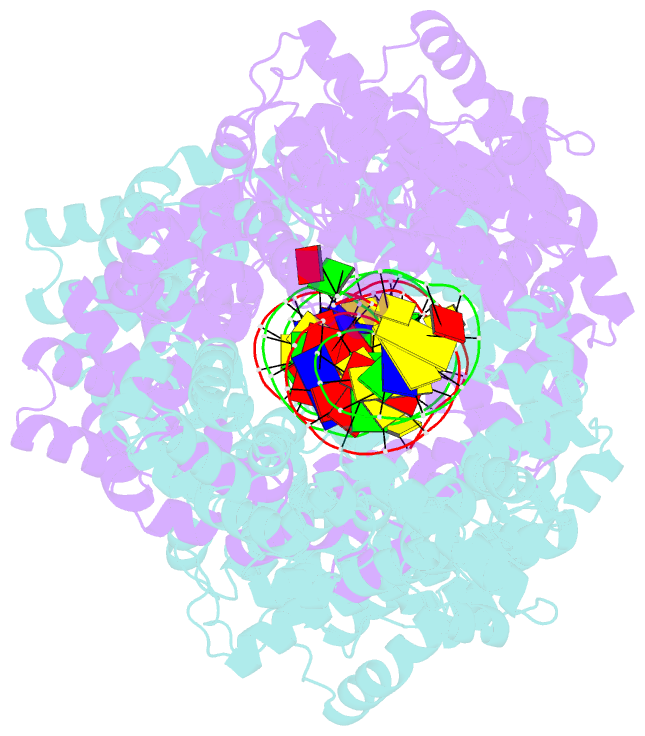
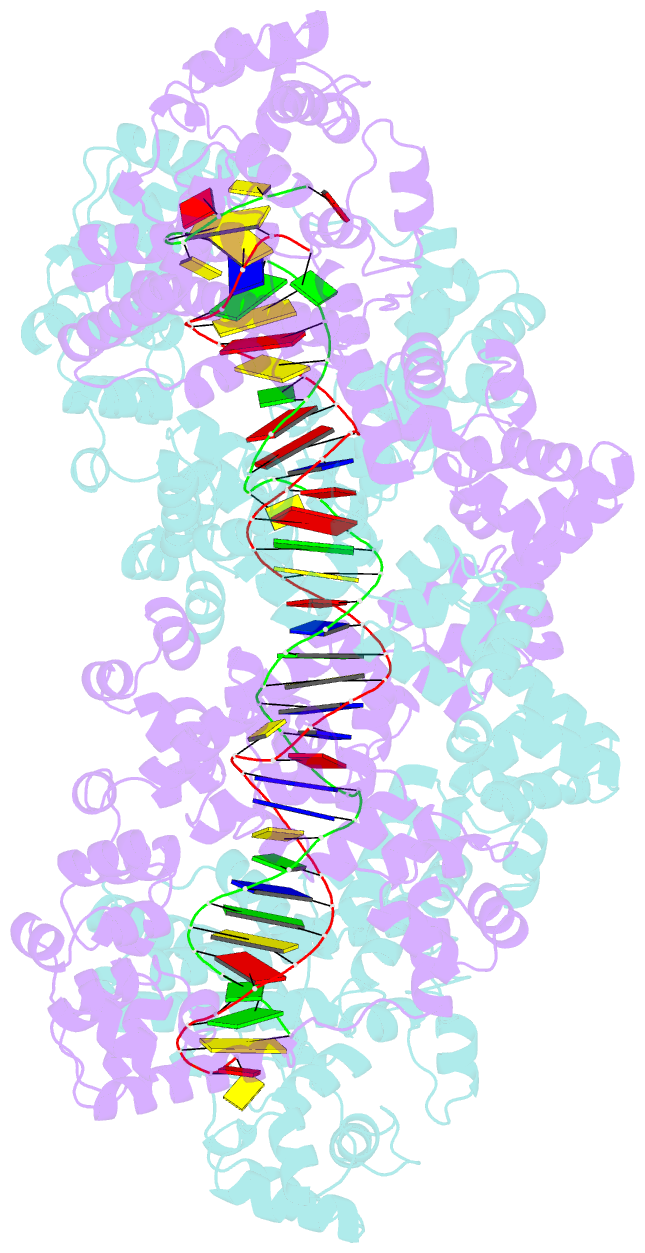
- Structure of m. kandleri topoisomerase v in complex with DNA. 38 base pair symmetric DNA complex
- Reference
- Osterman A, Mondragon A (2022): "Structures of topoisomerase V in complex with DNA reveal unusual DNA binding mode and novel relaxation mechanism." Elife, 11. doi: 10.7554/eLife.72702.
- Abstract
- Topoisomerase V is a unique topoisomerase that combines DNA repair and topoisomerase activities. The enzyme has an unusual arrangement, with a small topoisomerase domain followed by 12 tandem (HhH)2 domains, which include three AP lyase repair domains. The uncommon architecture of this enzyme bears no resemblance to any other known topoisomerase. Here we present structures of topoisomerase V in complex with DNA. The structures show that the (HhH)2 domains wrap around the DNA and in this manner appear to act as a processivity factor. There is a conformational change in the protein to expose the topoisomerase active site. The DNA bends sharply to enter the active site, which melts the DNA and probably facilitates relaxation. The structures show a DNA binding mode not observed before and provide information on the way this atypical topoisomerase relaxes DNA. In common with type IB enzymes, topoisomerase V relaxes DNA using a controlled rotation mechanism, but the structures show that topoisomerase V accomplishes this in different manner. Overall, the structures firmly establish that type IC topoisomerases form a distinct type of topoisomerases, with no similarities to other types at the sequence, structural, or mechanistic level. They represent a completely different solution to DNA relaxation.