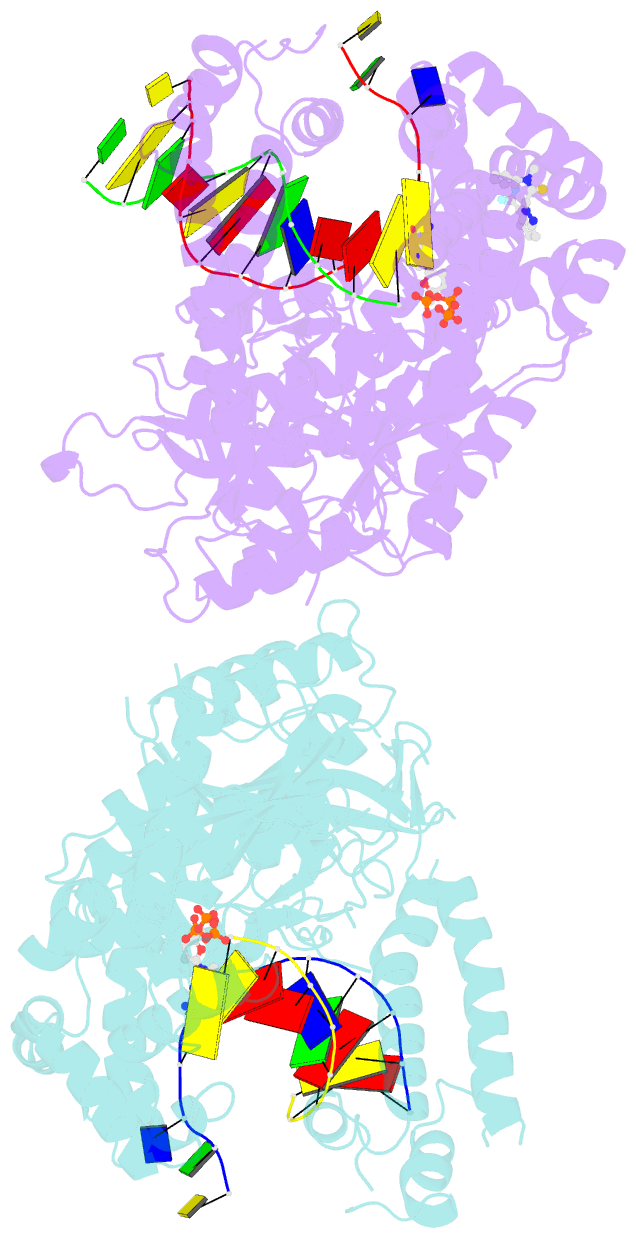
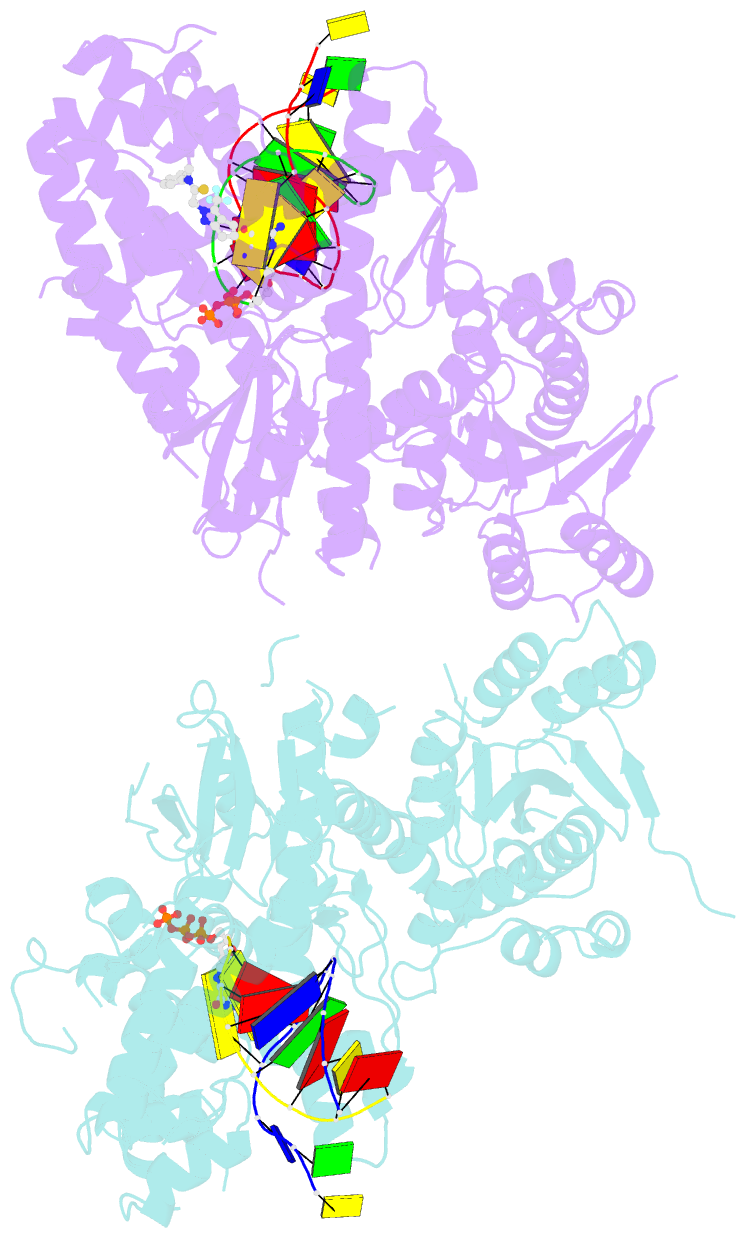
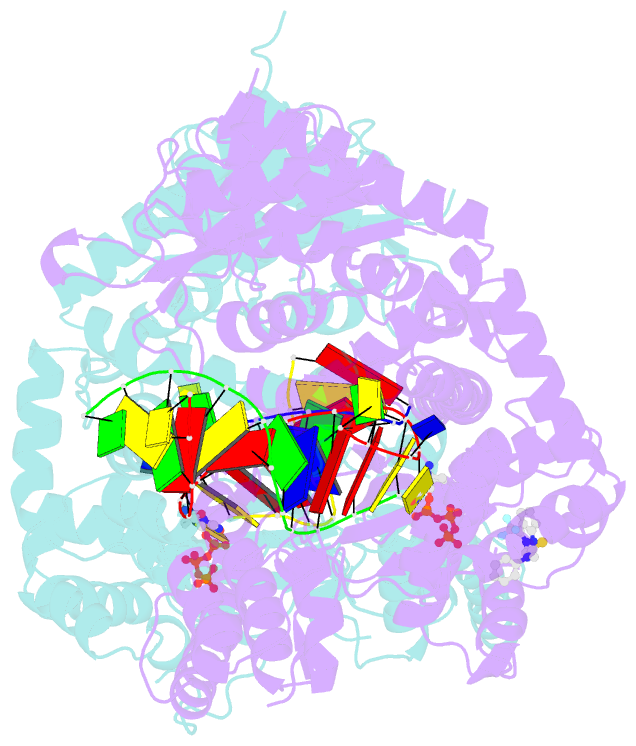
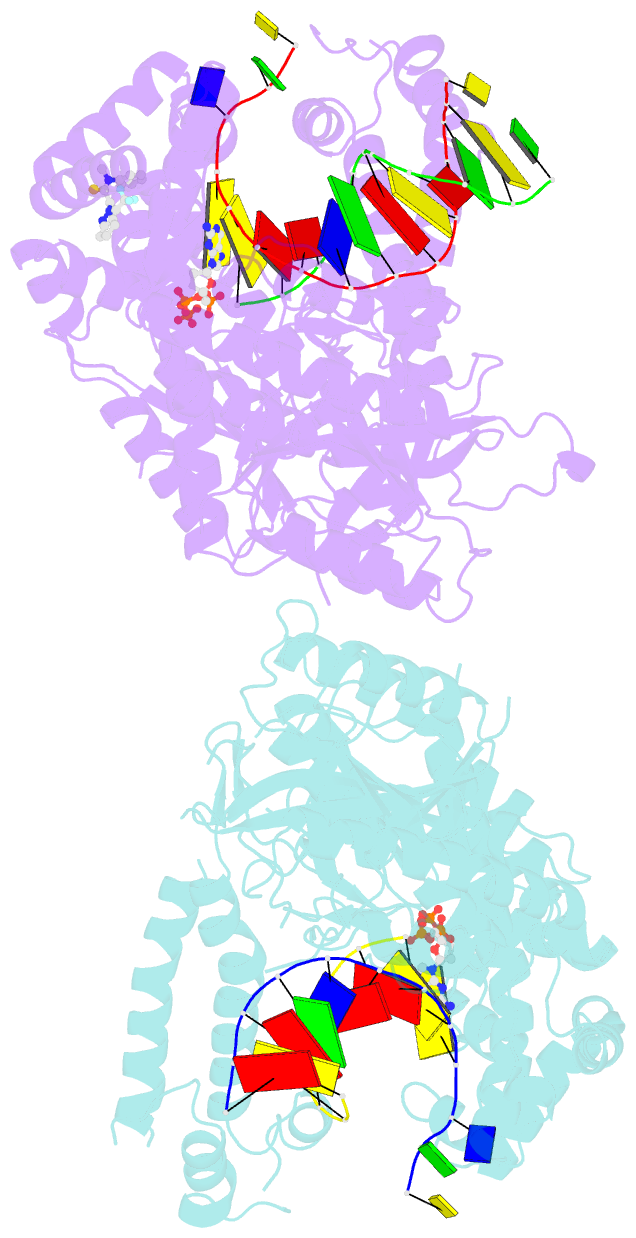
Summary information and primary citation
- PDB-id
- 8e23; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (2.59 Å)
- Summary
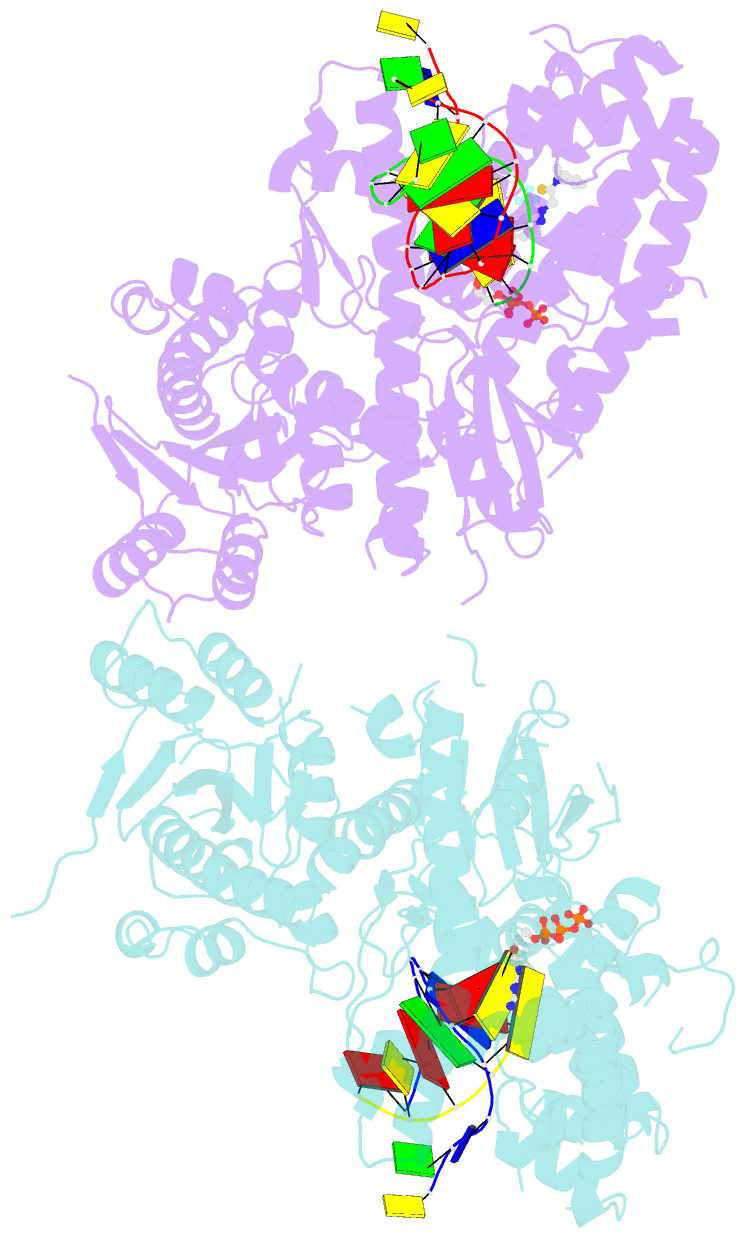
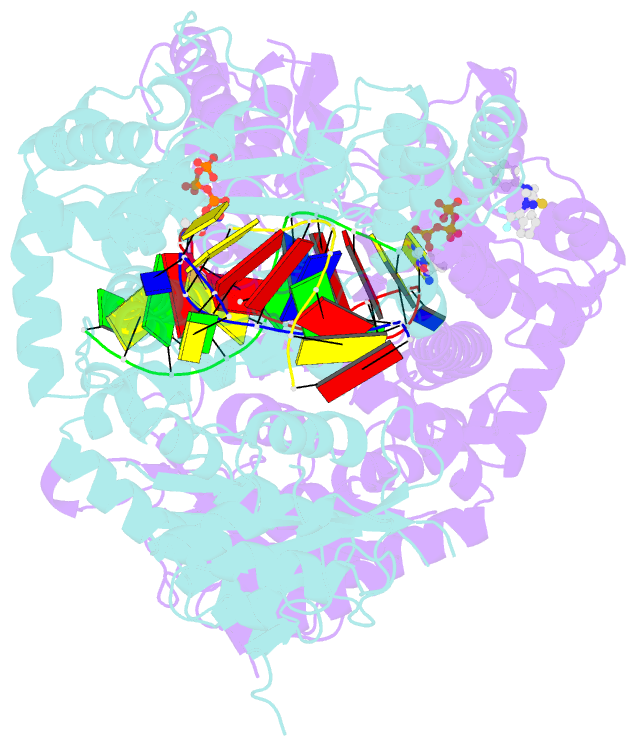
- Human DNA polymerase theta in complex with allosteric inhibitor
- Reference
- Bubenik M, Mader P, Mochirian P, Vallee F, Clark J, Truchon JF, Perryman AL, Pau V, Kurinov I, Zahn KE, Leclaire ME, Papp R, Mathieu MC, Hamel M, Duffy NM, Godbout C, Casas-Selves M, Falgueyret JP, Baruah PS, Nicolas O, Stocco R, Poirier H, Martino G, Fortin AB, Roulston A, Chefson A, Dorich S, St-Onge M, Patel P, Pellerin C, Ciblat S, Pinter T, Barabe F, El Bakkouri M, Parikh P, Gervais C, Sfeir A, Mamane Y, Morris SJ, Black WC, Sicheri F, Gallant M (2022): "Identification of RP-6685 , an Orally Bioavailable Compound that Inhibits the DNA Polymerase Activity of Pol theta." J.Med.Chem., 65, 13198-13215. doi: 10.1021/acs.jmedchem.2c00998.
- Abstract
- DNA polymerase theta (Polθ) is an attractive synthetic lethal target for drug discovery, predicted to be efficacious against breast and ovarian cancers harboring BRCA-mutant alleles. Here, we describe our hit-to-lead efforts in search of a selective inhibitor of human Polθ (encoded by POLQ). A high-throughput screening campaign of 350,000 compounds identified an 11 micromolar hit, giving rise to the N2-substituted fused pyrazolo series, which was validated by biophysical methods. Structure-based drug design efforts along with optimization of cellular potency and ADME ultimately led to the identification of