Summary information and primary citation
- PDB-id
- 8e6z; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription, transferase-DNA-RNA
- Method
- cryo-EM (4.1 Å)
- Summary
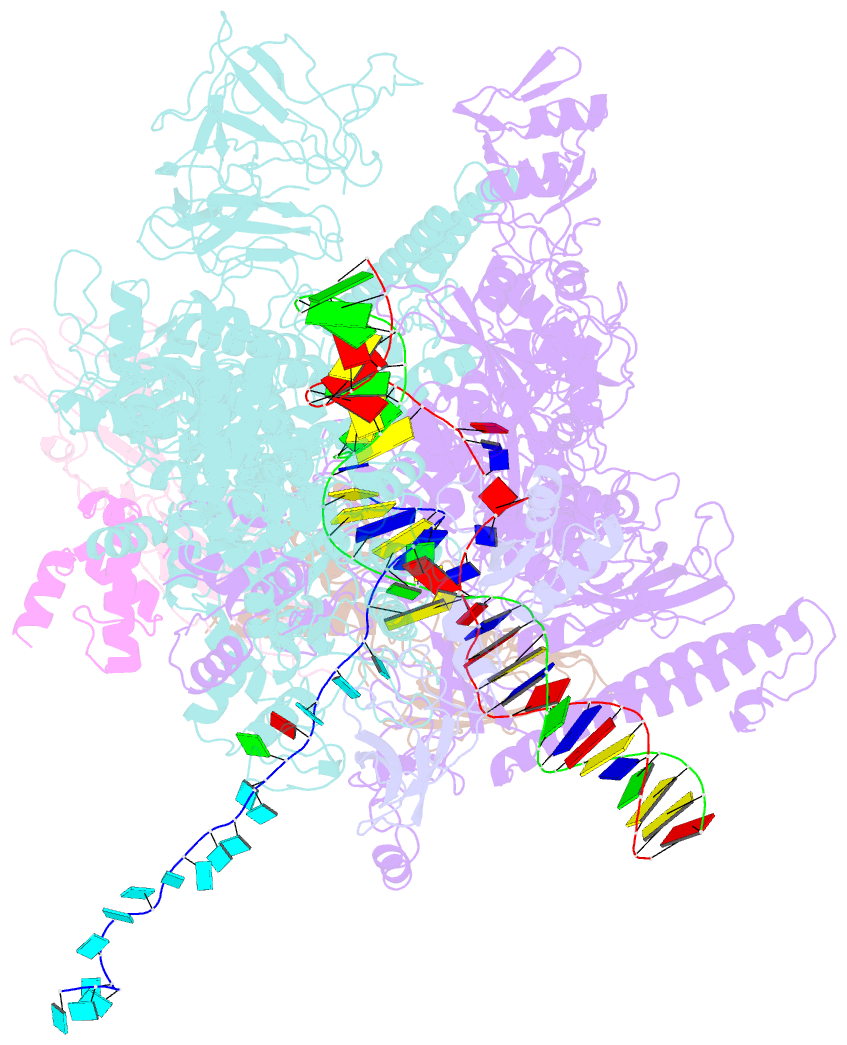
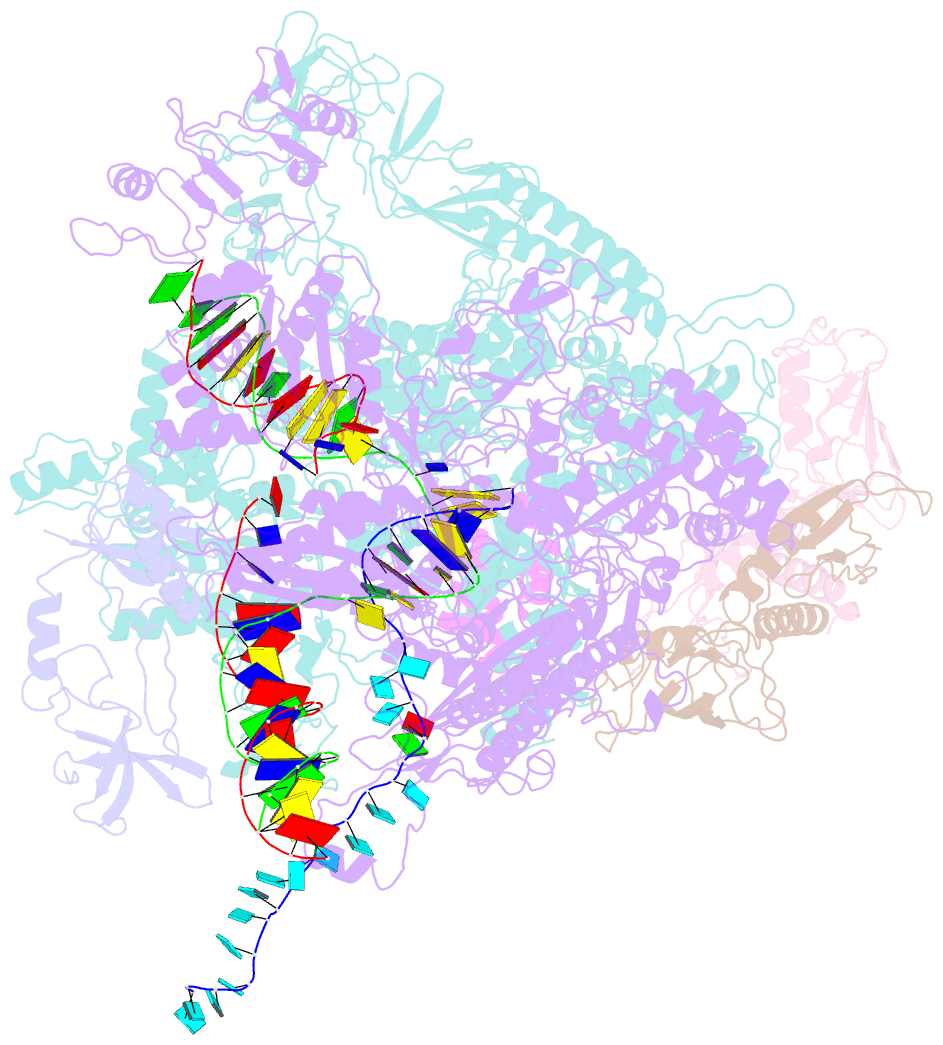
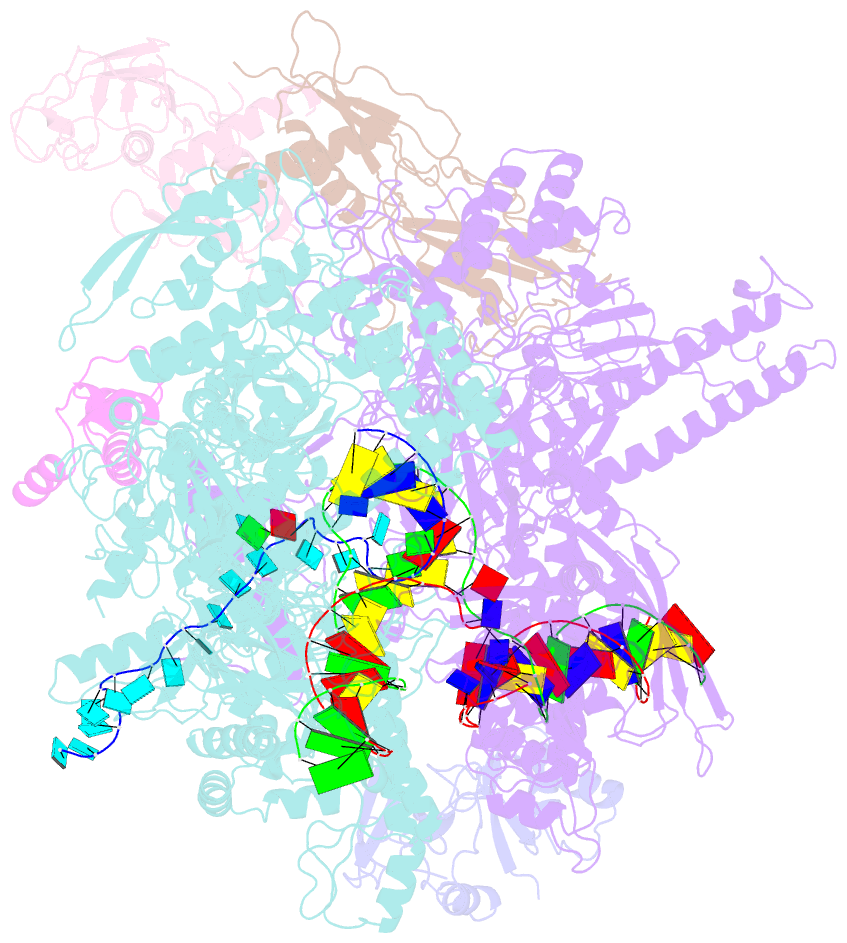
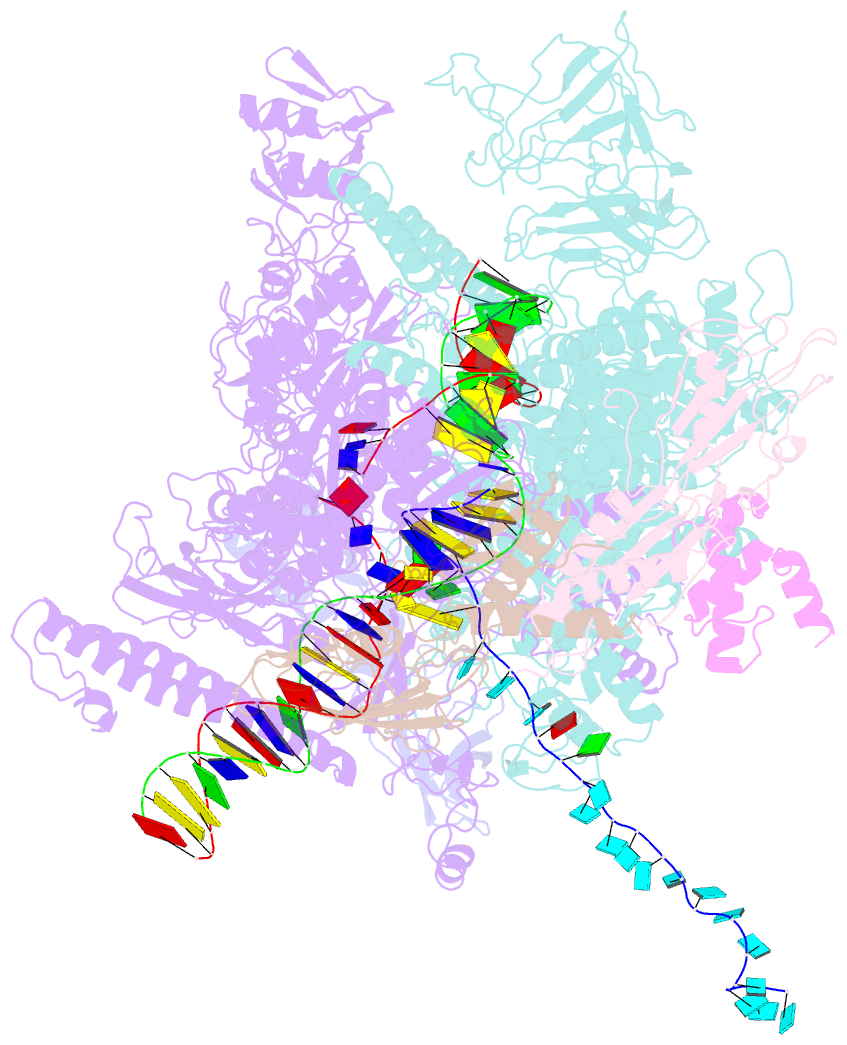
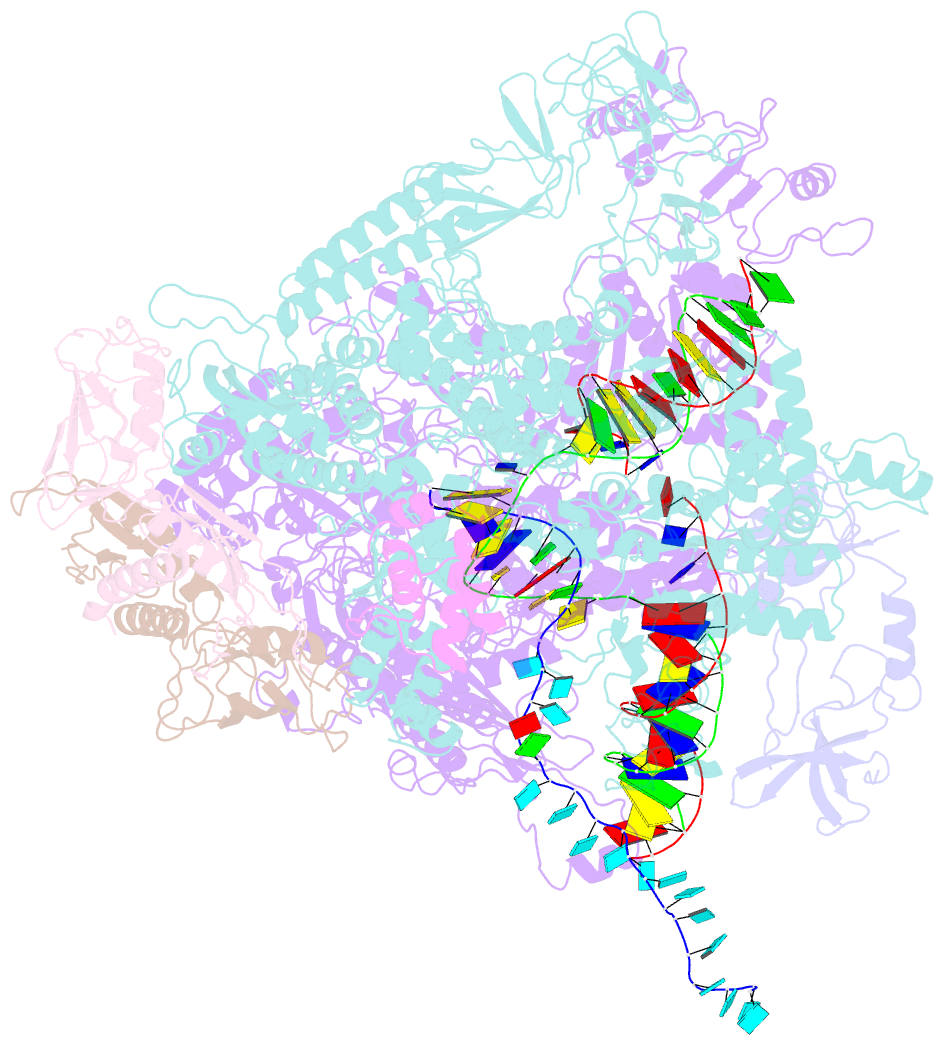
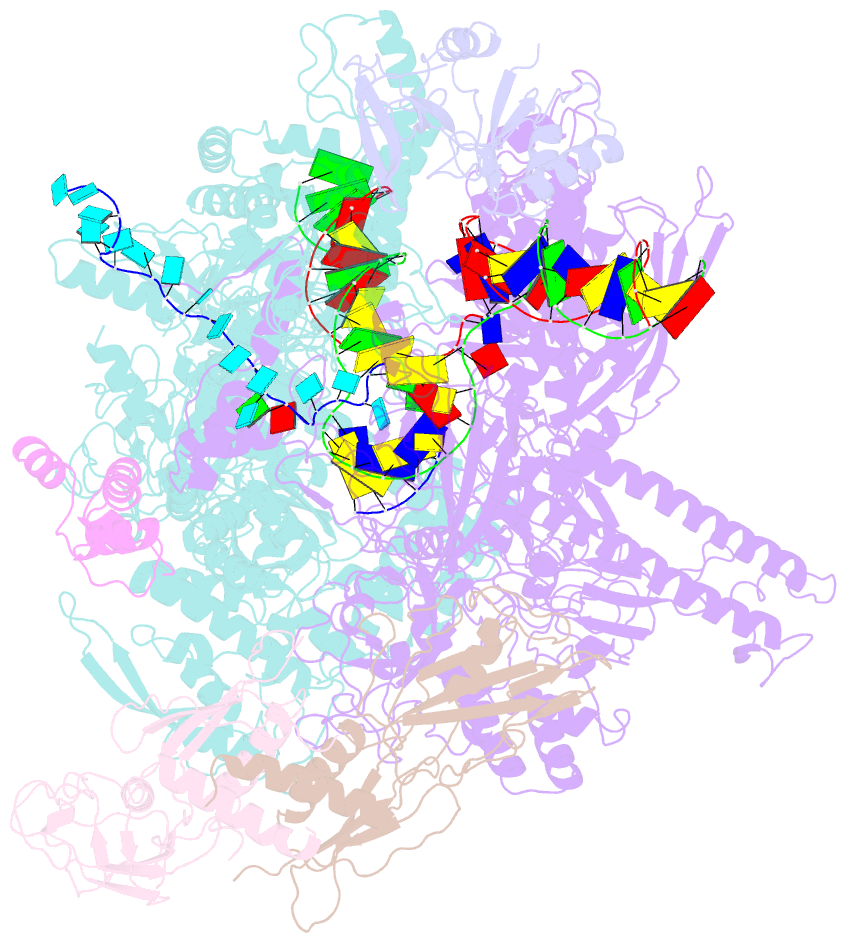
- Escherichia coli rho-dependent transcription pre-termination complex containing 18 nt long RNA spacer, dc75 rut mimic RNA, mg-adp-bef3, and nusg; tec part
- Reference
- Molodtsov V, Wang C, Firlar E, Kaelber JT, Ebright RH (2023): "Structural basis of Rho-dependent transcription termination." Nature, 614, 367-374. doi: 10.1038/s41586-022-05658-1.
- Abstract
- Rho is a ring-shaped hexameric ATP-dependent molecular motor. Together with the transcription elongation factor NusG, Rho mediates factor-dependent transcription termination and transcription-translation-coupling quality control in Escherichia coli1-4. Here we report the preparation of complexes that are functional in factor-dependent transcription termination from Rho, NusG, RNA polymerase (RNAP), and synthetic nucleic acid scaffolds, and we report cryogenic electron microscopy structures of the complexes. The structures show that functional factor-dependent pre-termination complexes contain a closed-ring Rho hexamer; have RNA threaded through the central channel of Rho; have 60 nucleotides of RNA interacting sequence-specifically with the exterior of Rho and 6 nucleotides of RNA interacting sequence-specifically with the central channel of Rho; have Rho oriented relative to RNAP such that ATP-dependent translocation by Rho exerts mechanical force on RNAP; and have NusG bridging Rho and RNAP. The results explain five decades of research on Rho and provide a foundation for understanding Rho's function.