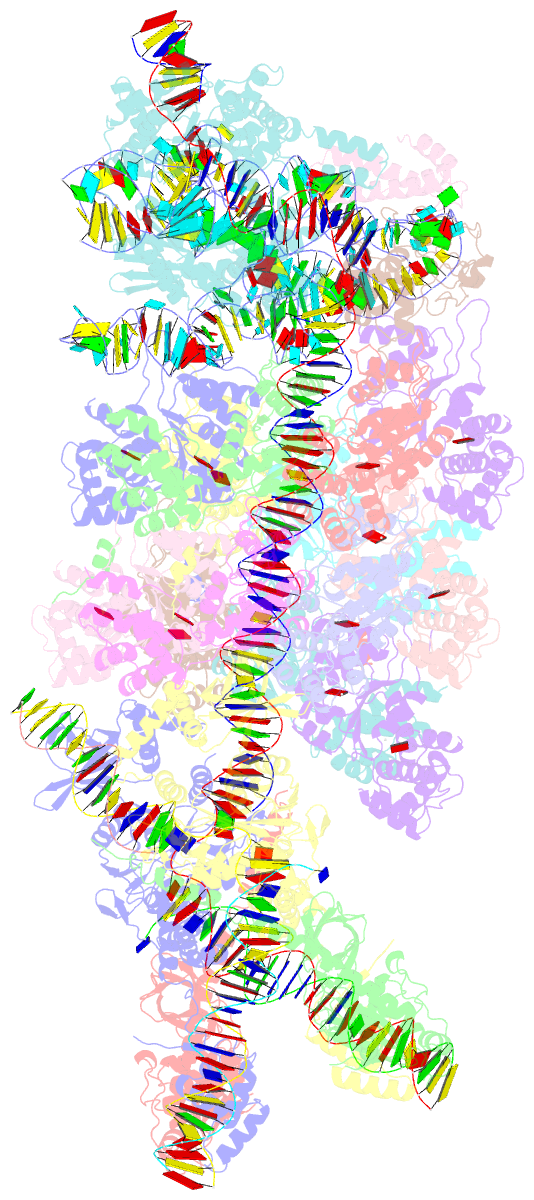
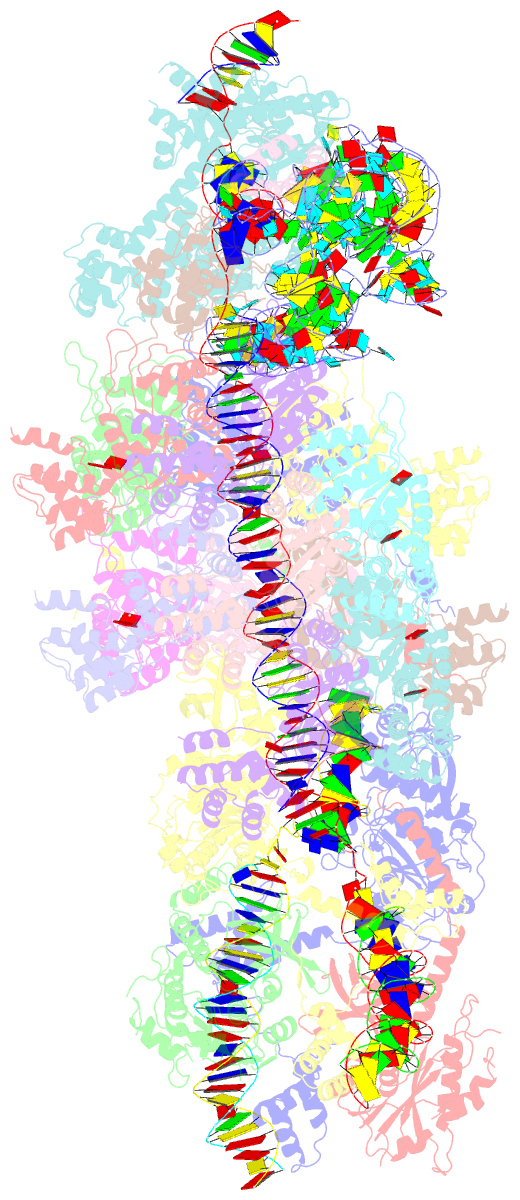
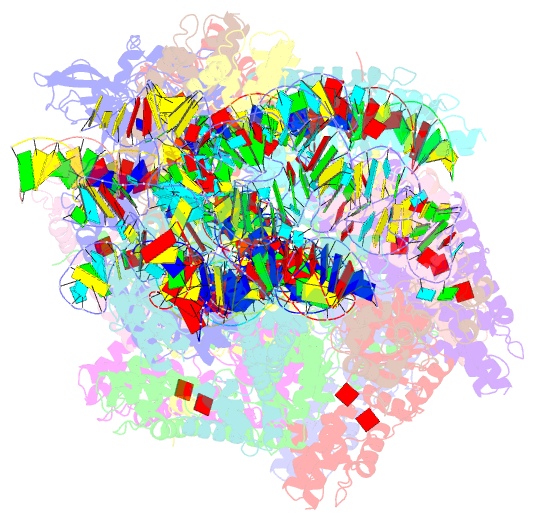
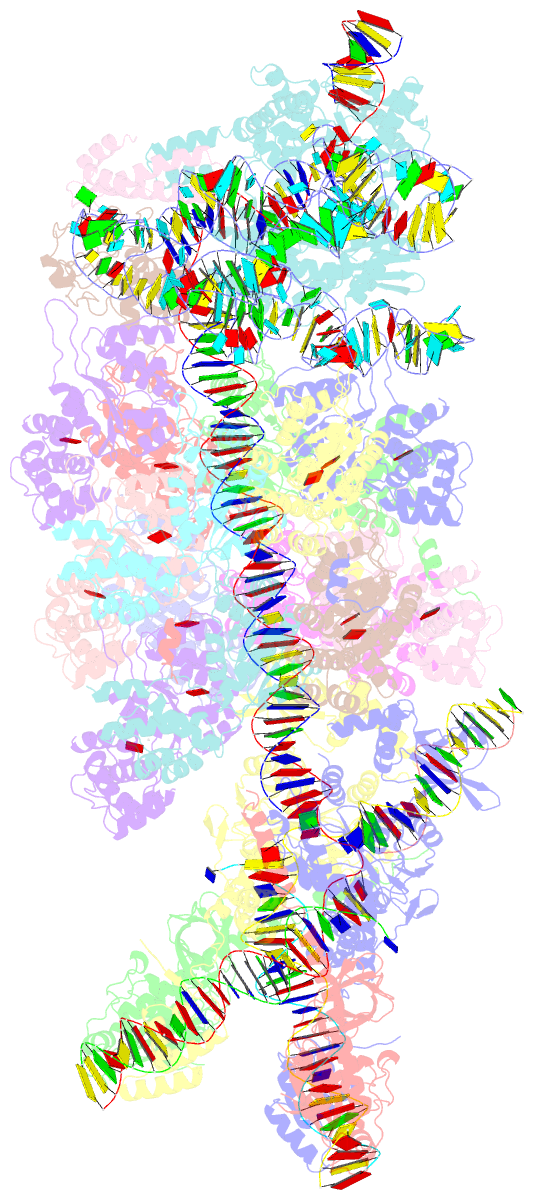
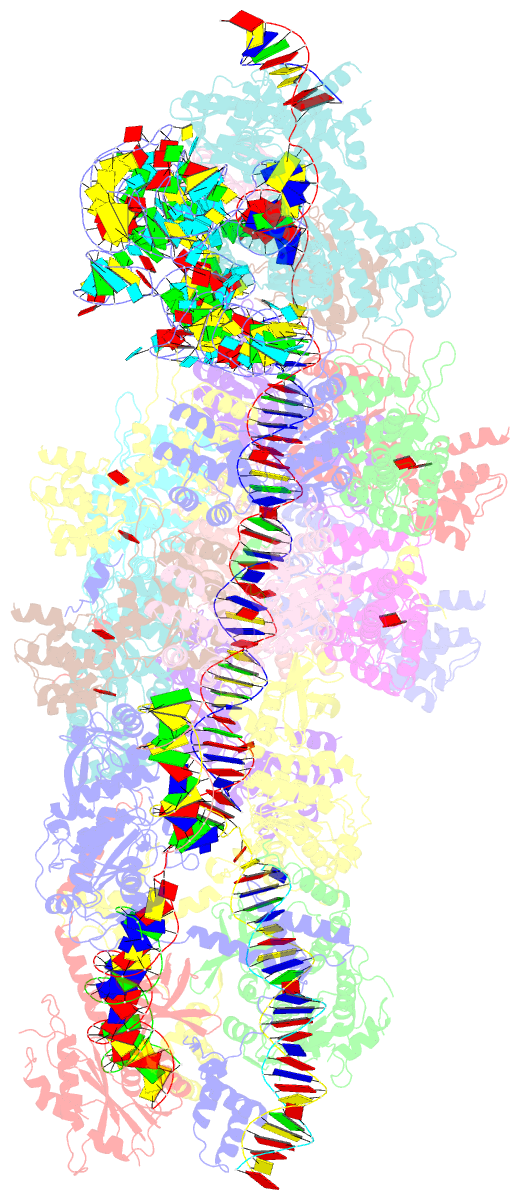
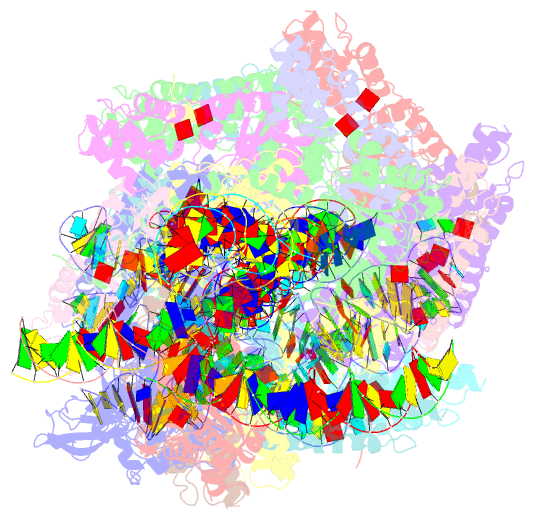
Summary information and primary citation
- PDB-id
- 8ea4; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-RNA-DNA
- Method
- cryo-EM (3.0 Å)
- Summary
- V-k cast transpososome from scytonema hofmanni, minor configuration
- Reference
- Park JU, Tsai AW, Rizo AN, Truong VH, Wellner TX, Schargel RD, Kellogg EH (2023): "Structures of the holo CRISPR RNA-guided transposon integration complex." Nature, 613, 775-782. doi: 10.1038/s41586-022-05573-5.
- Abstract
- CRISPR-associated transposons (CAST) are programmable mobile genetic elements that insert large DNA cargo using an RNA-guided mechanism1-3. CAST elements contain multiple conserved genes, including: a CRISPR effector (Cas12k or Cascade), a AAA+ regulator (TnsC), a transposase (TnsA/TnsB), and a target site associated factor (TniQ). These components are thought to cooperatively integrate DNA via formation of a multisubunit transposition integration complex (transpososome). Here we reconstitute the approximately 1 MDa type V-K CAST transpososome from Scytonema hofmannii (ShCAST) and determined the structure to near-atomic resolution (3.5 Å) using single particle cryo-EM. Transpososome architecture reveals modular association between components. Cas12k forms a complex with ribosomal subunit S15 and TniQ, stabilizing formation of a full R-loop. TnsC has dedicated interaction interfaces with TniQ and TnsB. Interestingly, we observe novel TnsC-TnsB interactions at the C-terminal face of TnsC, which contribute to stimulation of ATPase activity. Although the TnsC oligomeric assembly slightly deviates from the helical configuration found in isolation, the TnsC-bound target DNA conformation differs dramatically in the transpososome. As a consequence, TnsC makes new protein-DNA interactions throughout the transpososome that are important for transposition activity. Finally, we discover two distinct transpososome populations which differ in their DNA contacts near TniQ. This suggests that associations with the CRISPR effector can be flexible. This ShCAST transpososome structure significantly enhances our understanding of CAST transposition systems and suggests avenues for improving CAST transposition for precision genome-editing applications.