Summary information and primary citation
- PDB-id
- 8edg; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- recombination-DNA
- Method
- cryo-EM (4.64 Å)
- Summary
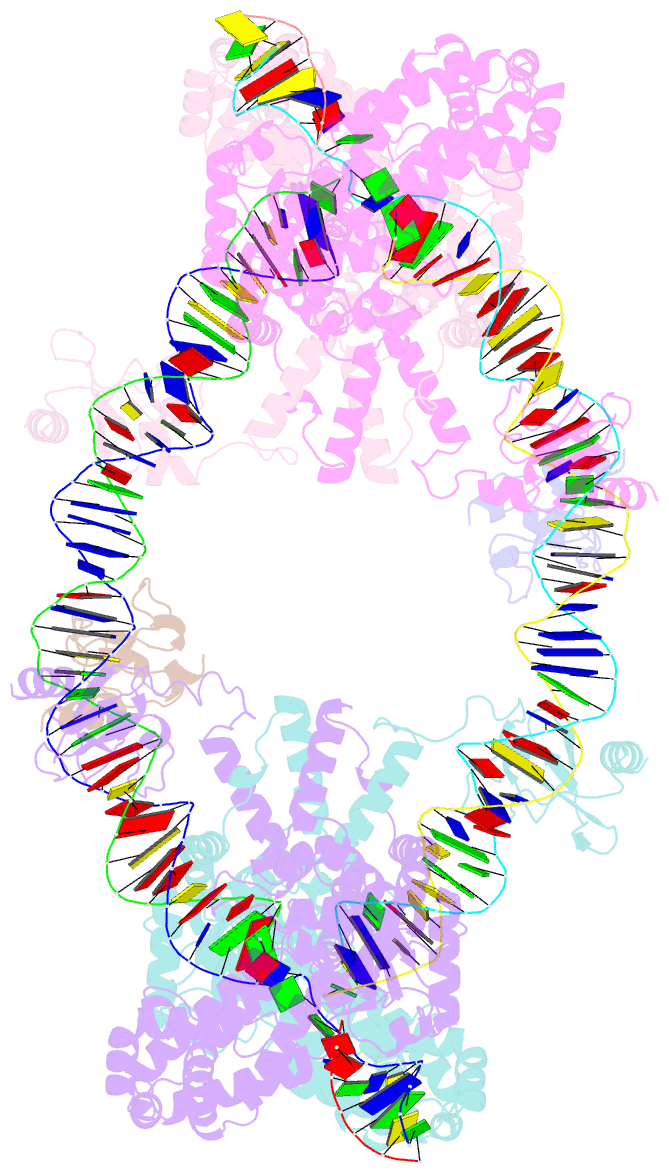
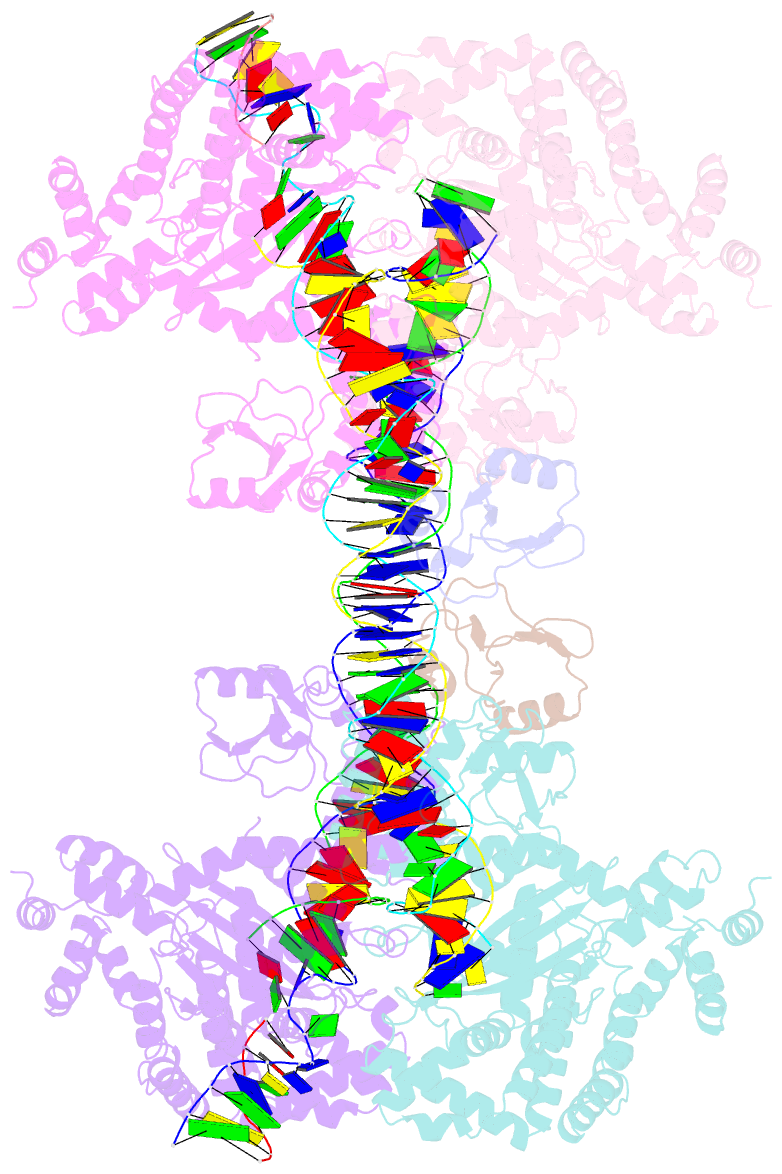
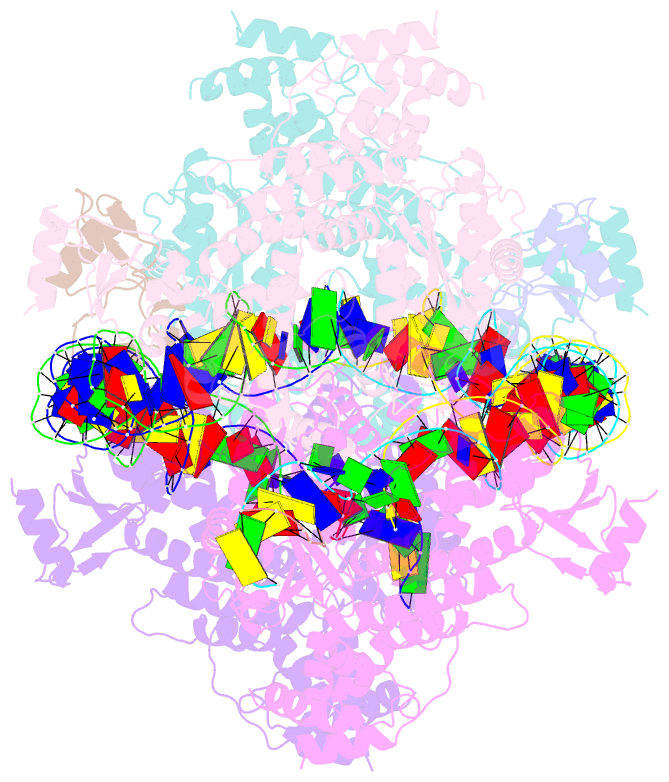
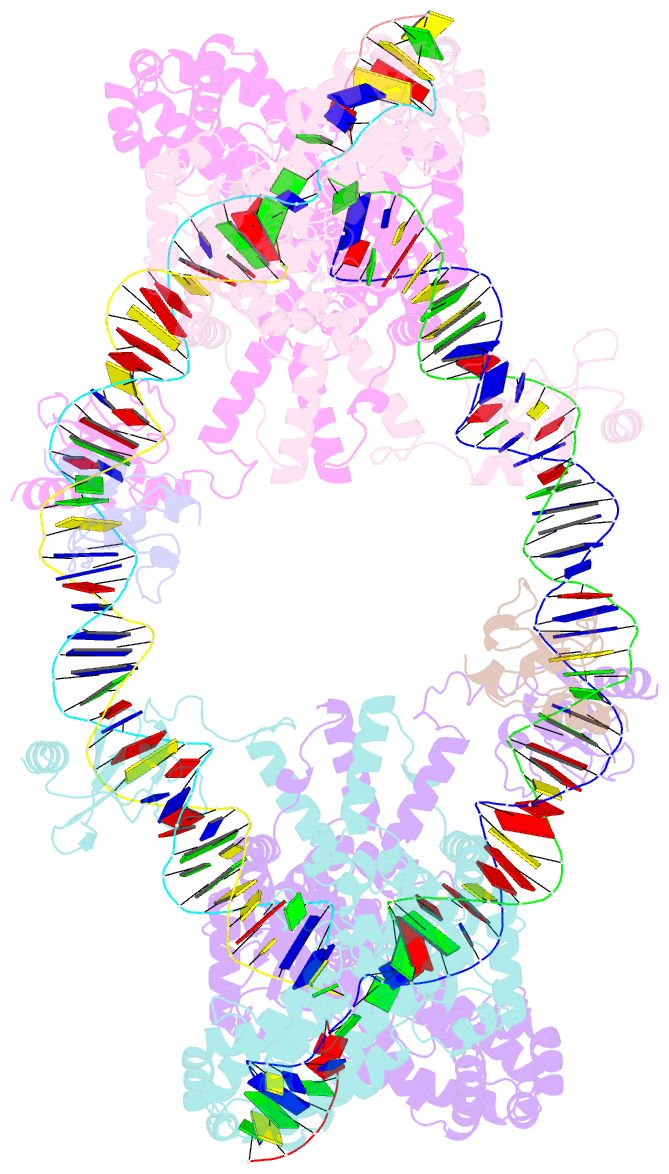
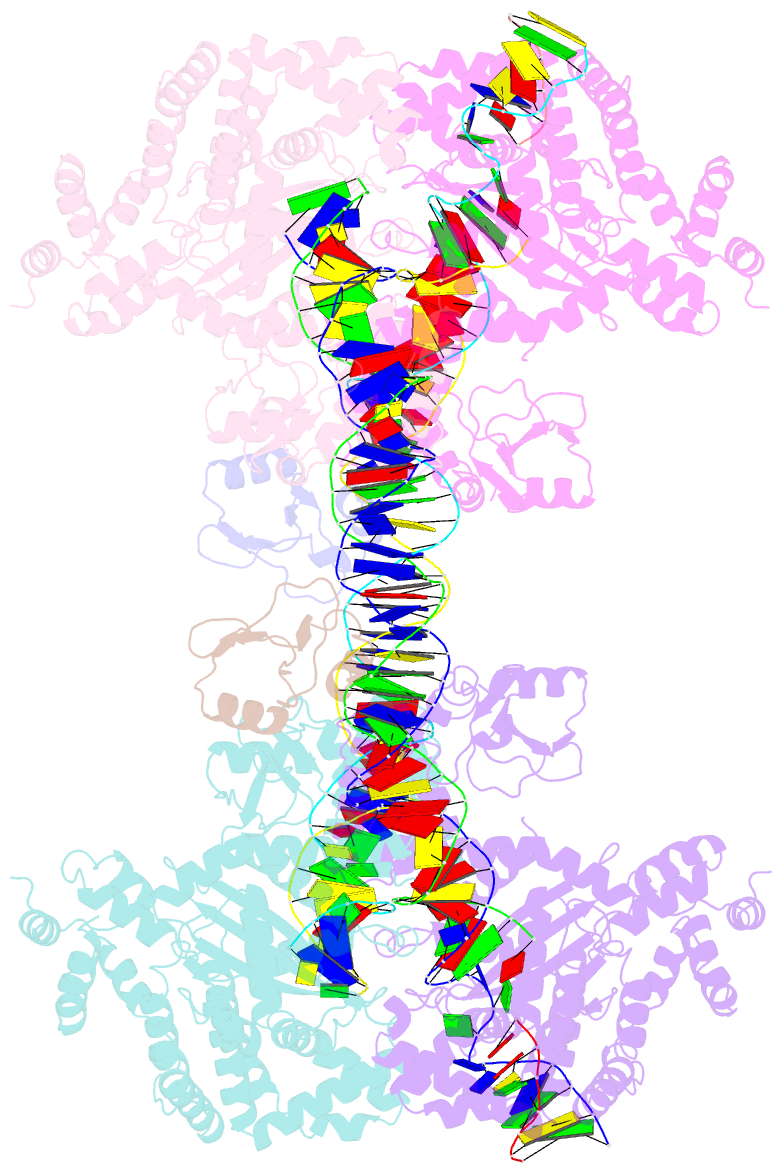
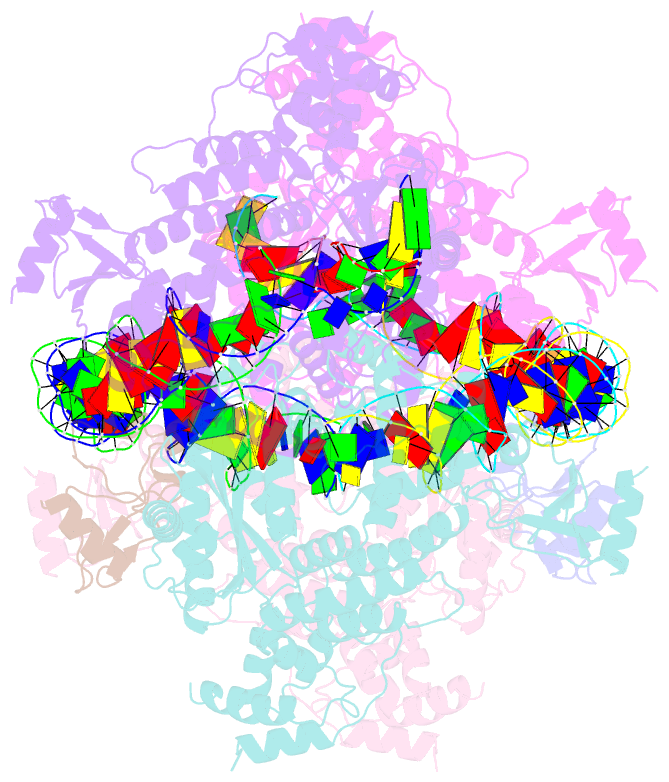
- cryo-EM structure of the hermes transposase bound to two left-ends of its DNA transposon
- Reference
- Lannes L, Furman CM, Hickman AB, Dyda F (2023): "Zinc-finger BED domains drive the formation of the active Hermes transpososome by asymmetric DNA binding." Nat Commun, 14, 4470. doi: 10.1038/s41467-023-40210-3.
- Abstract
- The Hermes DNA transposon is a member of the eukaryotic hAT superfamily, and its transposase forms a ring-shaped tetramer of dimers. Our investigation, combining biochemical, crystallography and cryo-electron microscopy, and in-cell assays, shows that the full-length Hermes octamer extensively interacts with its transposon left-end through multiple BED domains of three Hermes protomers contributed by three dimers explaining the role of the unusual higher-order assembly. By contrast, the right-end is bound to no BED domains at all. Thus, this work supports a model in which Hermes multimerizes to gather enough BED domains to find its left-end among the abundant genomic DNA, facilitating the subsequent interaction with the right-end.