Summary information and primary citation
- PDB-id
- 8efk; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- cryo-EM (3.0 Å)
- Summary
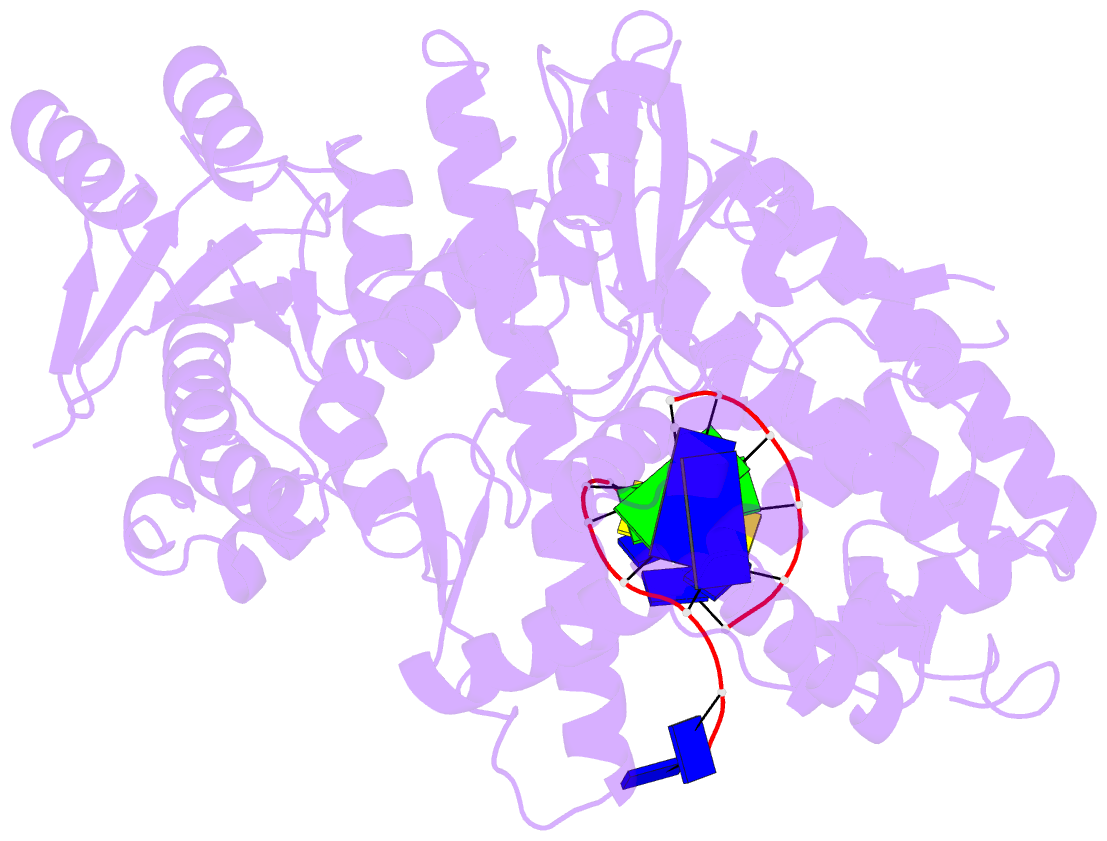
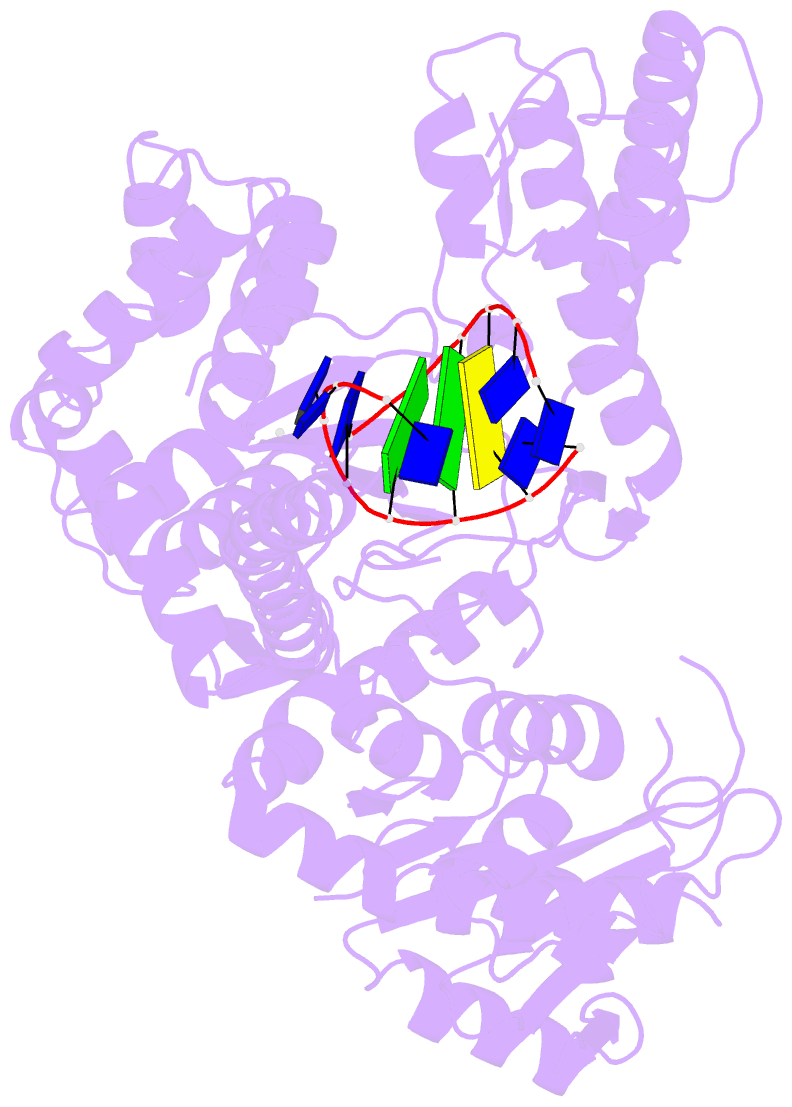
- Structure of lates calcarifer DNA polymerase theta polymerase domain with hairpin DNA
- Reference
- Li C, Zhu H, Jin S, Maksoud LM, Jain N, Sun J, Gao Y (2023): "Structural basis of DNA polymerase theta mediated DNA end joining." Nucleic Acids Res., 51, 463-474. doi: 10.1093/nar/gkac1201.
- Abstract
- DNA polymerase θ (Pol θ) plays an essential role in the microhomology-mediated end joining (MMEJ) pathway for repairing DNA double-strand breaks. However, the mechanisms by which Pol θ recognizes microhomologous DNA ends and performs low-fidelity DNA synthesis remain unclear. Here, we present cryo-electron microscope structures of the polymerase domain of Lates calcarifer Pol θ with long and short duplex DNA at up to 2.4 Å resolution. Interestingly, Pol θ binds to long and short DNA substrates similarly, with extensive interactions around the active site. Moreover, Pol θ shares a similar active site as high-fidelity A-family polymerases with its finger domain well-closed but differs in having hydrophilic residues surrounding the nascent base pair. Computational simulations and mutagenesis studies suggest that the unique insertion loops of Pol θ help to stabilize short DNA binding and assemble the active site for MMEJ repair. Taken together, our results illustrate the structural basis of Pol θ-mediated MMEJ.