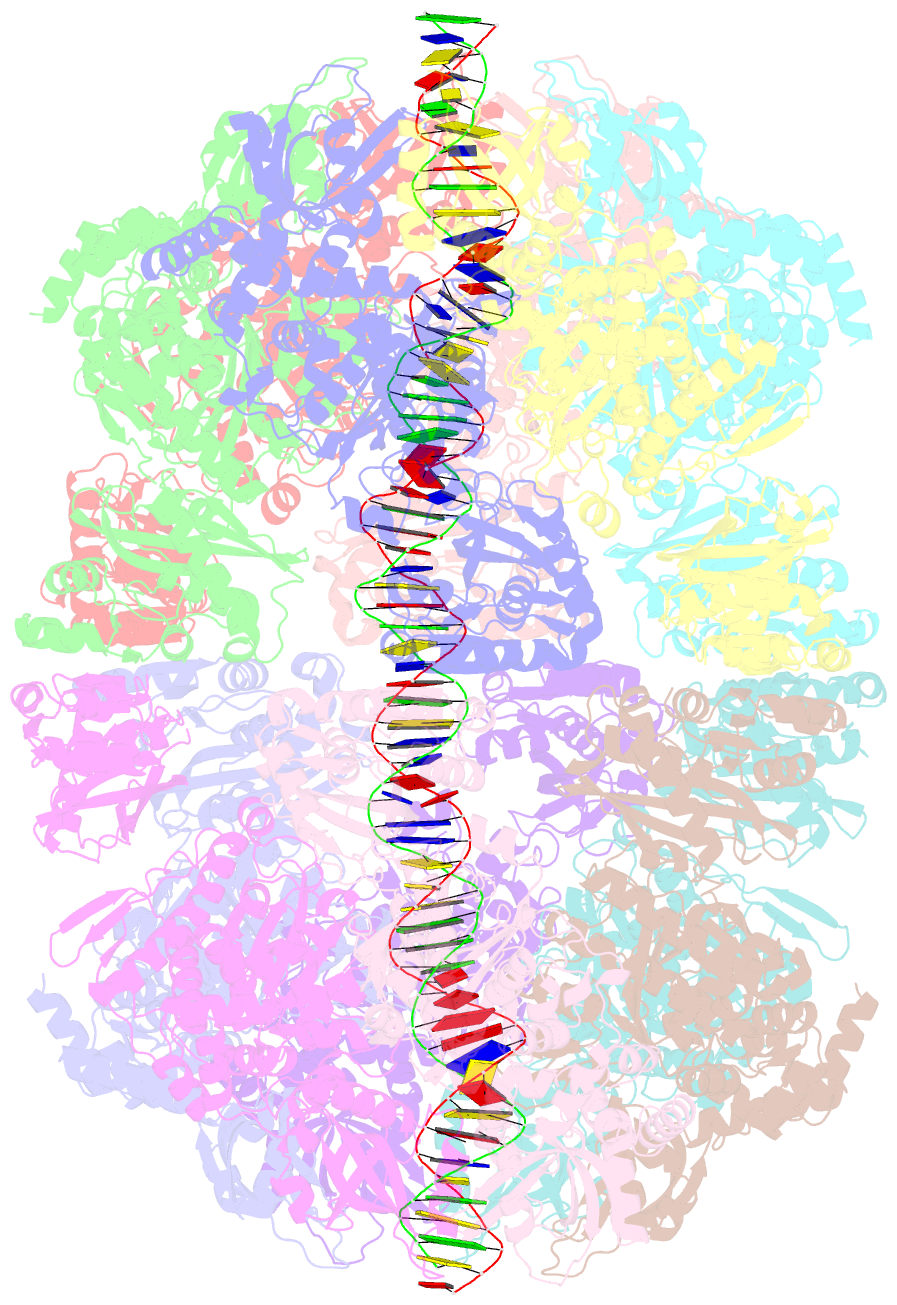
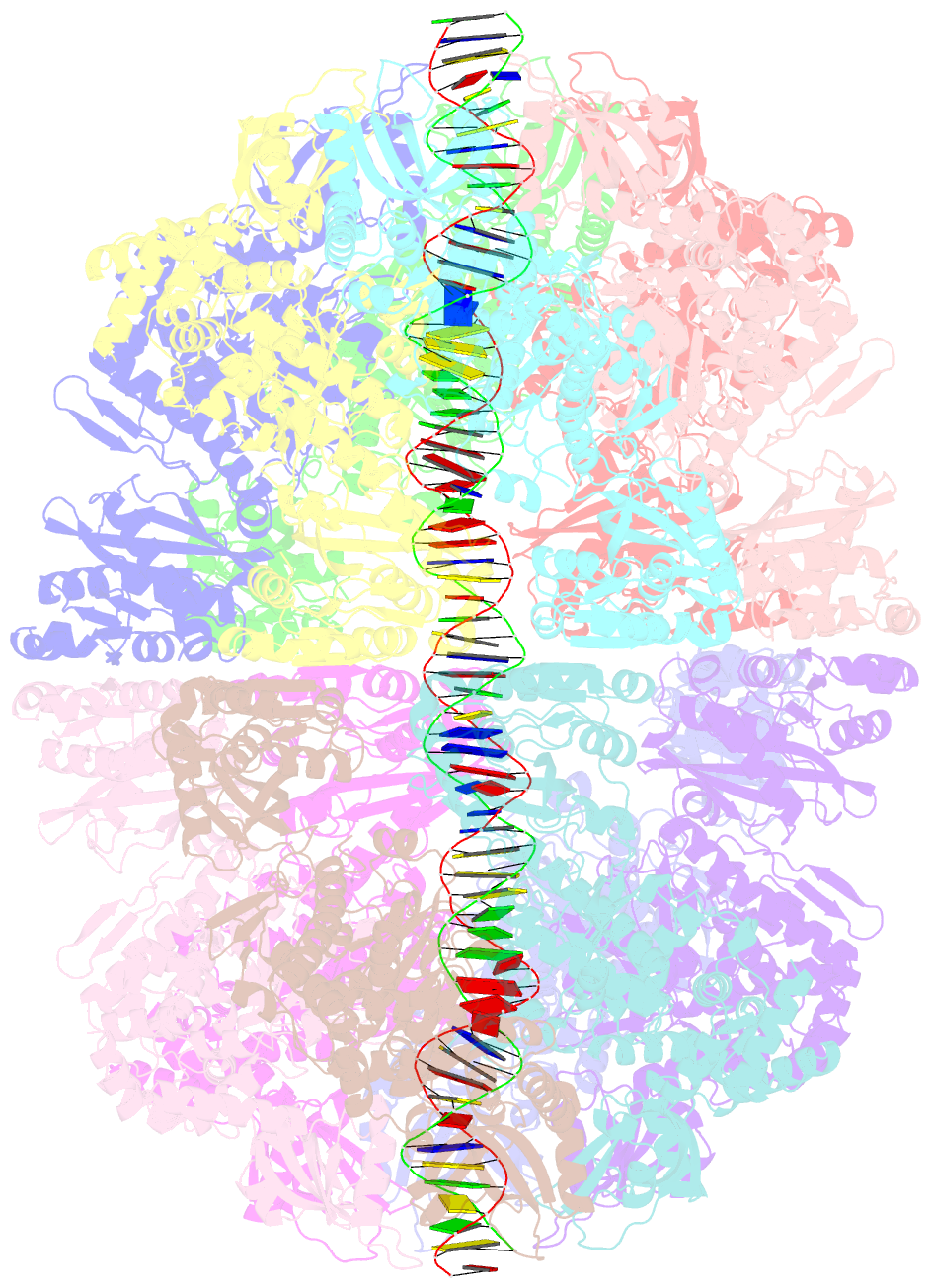
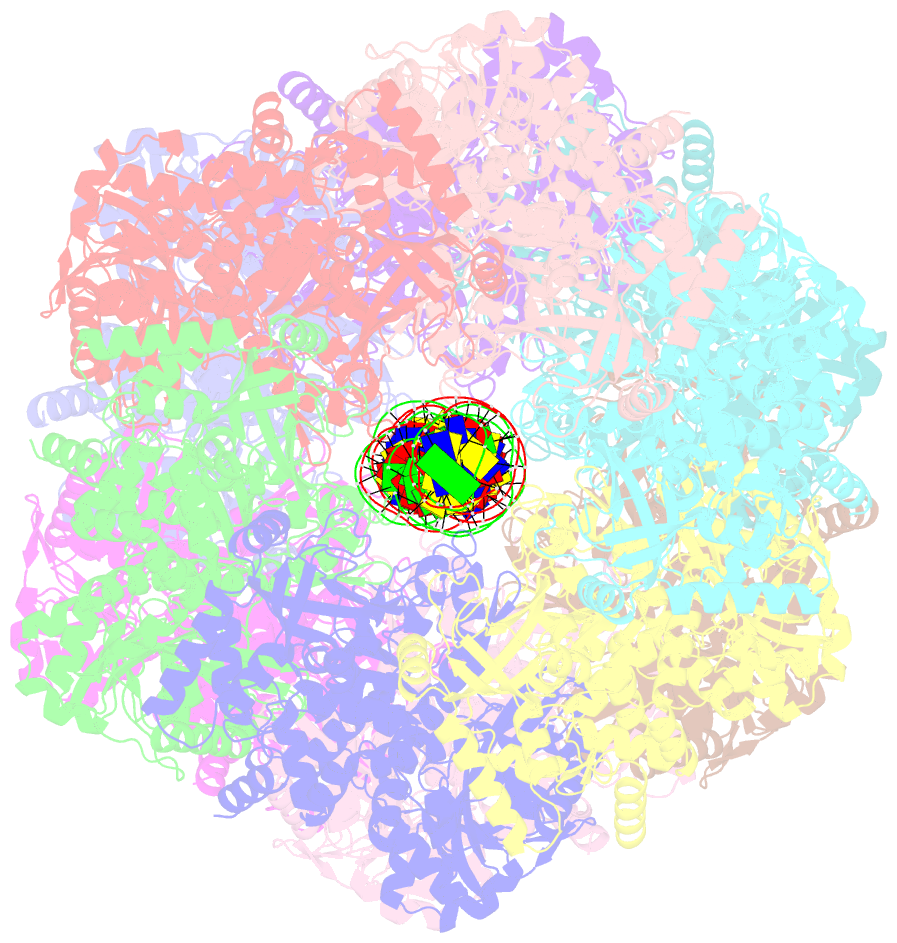
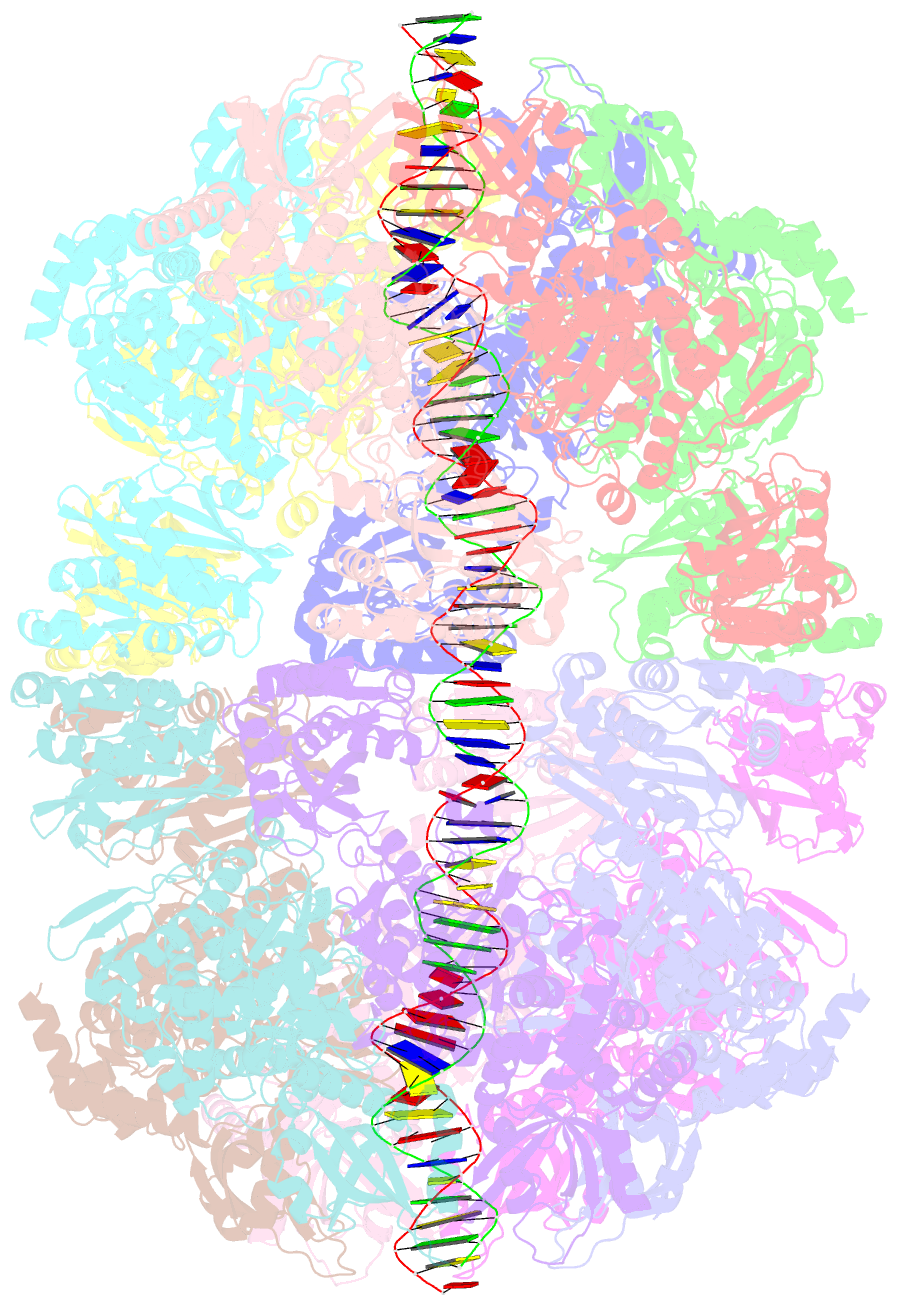
Summary information and primary citation
- PDB-id
- 8emh; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- antimicrobial protein
- Method
- cryo-EM (3.63 Å)
- Summary
- Cryoem characterization of a unique aaa+ brxl phage restriction factor
- Reference
- Shen BW, Doyle LA, Werther R, Westburg AA, Bies DP, Walter SI, Luyten YA, Morgan RD, Stoddard BL, Kaiser BK (2023): "Structure, substrate binding and activity of a unique AAA+ protein: the BrxL phage restriction factor." Nucleic Acids Res., 51, 3513-3528. doi: 10.1093/nar/gkad083.
- Abstract
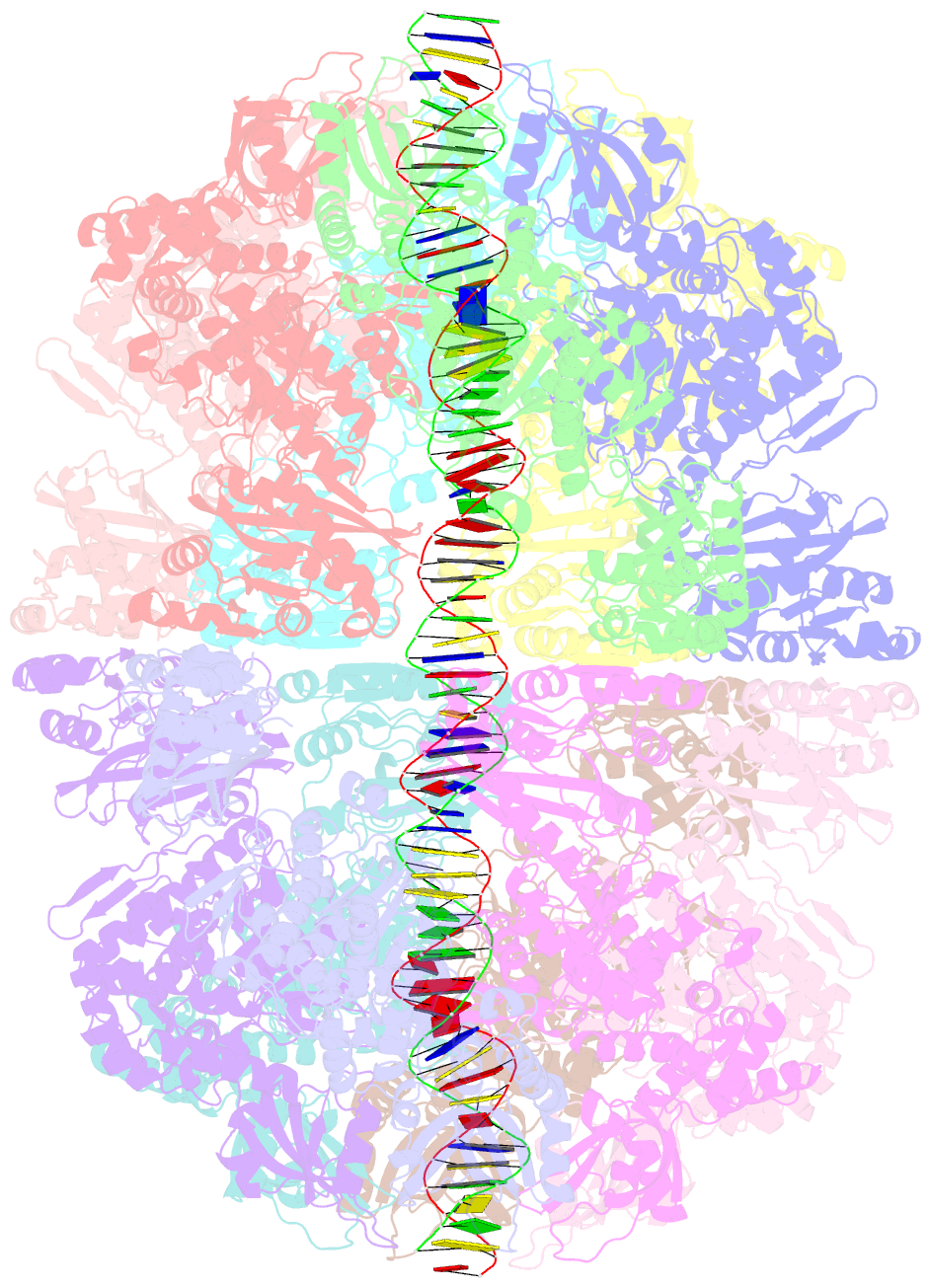
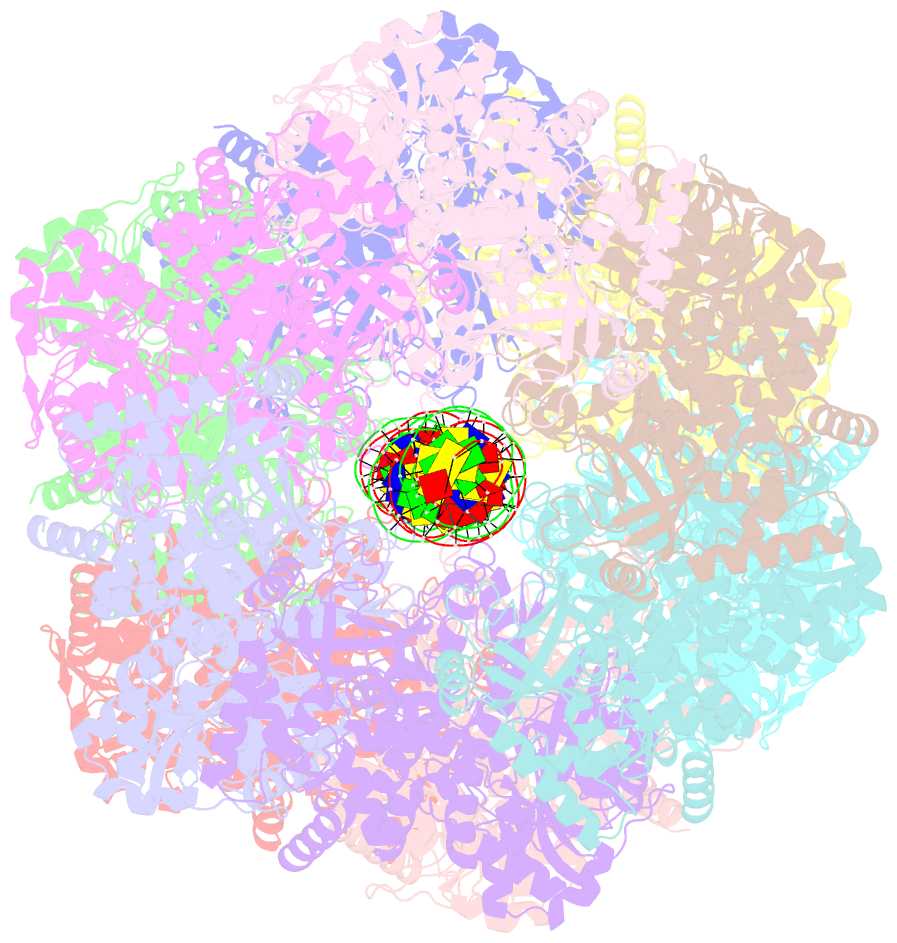
- Bacteriophage exclusion ('BREX') systems are multi-protein complexes encoded by a variety of bacteria and archaea that restrict phage by an unknown mechanism. One BREX factor, termed BrxL, has been noted to display sequence similarity to various AAA+ protein factors including Lon protease. In this study we describe multiple CryoEM structures of BrxL that demonstrate it to be a chambered, ATP-dependent DNA binding protein. The largest BrxL assemblage corresponds to a dimer of heptamers in the absence of bound DNA, versus a dimer of hexamers when DNA is bound in its central pore. The protein displays DNA-dependent ATPase activity, and ATP binding promotes assembly of the complex on DNA. Point mutations within several regions of the protein-DNA complex alter one or more in vitro behaviors and activities, including ATPase activity and ATP-dependent association with DNA. However, only the disruption of the ATPase active site fully eliminates phage restriction, indicating that other mutations can still complement BrxL function within the context of an otherwise intact BREX system. BrxL displays significant structural homology to MCM subunits (the replicative helicase in archaea and eukaryotes), implying that it and other BREX factors may collaborate to disrupt initiation of phage DNA replication.