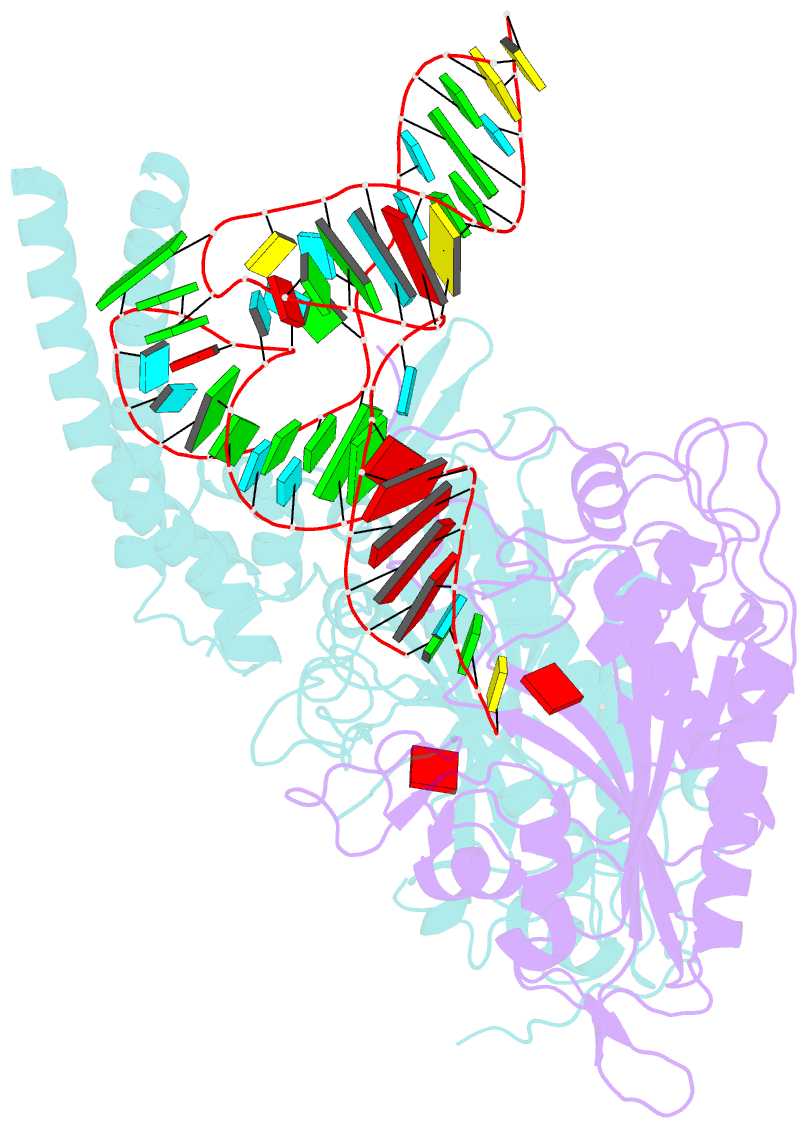
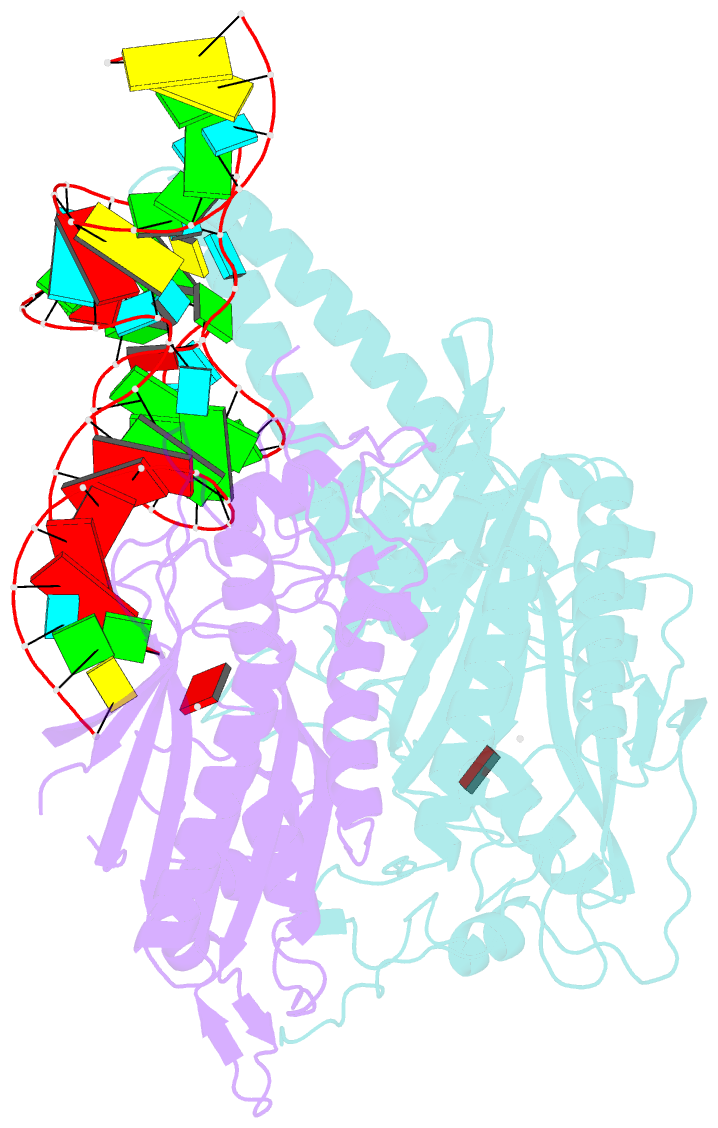
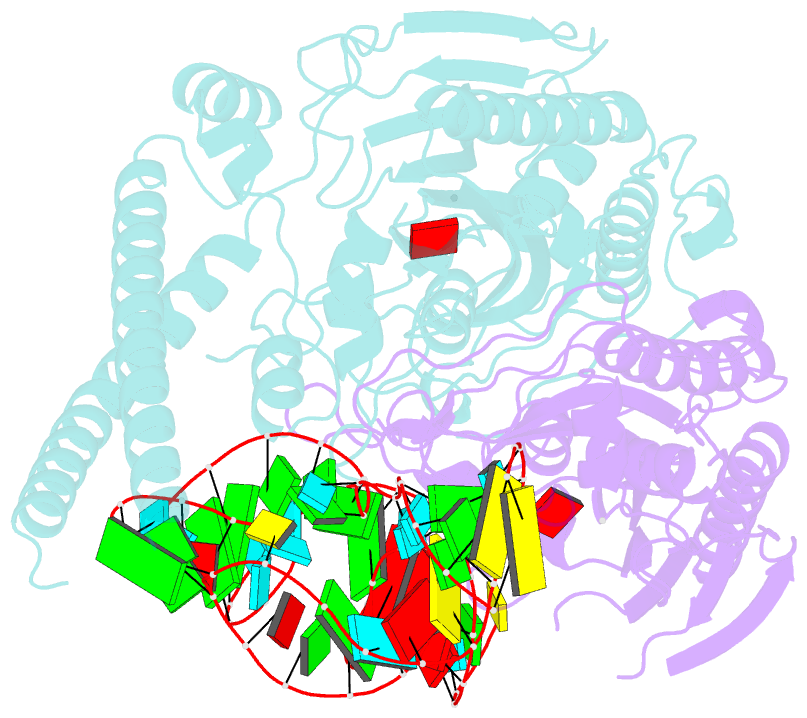
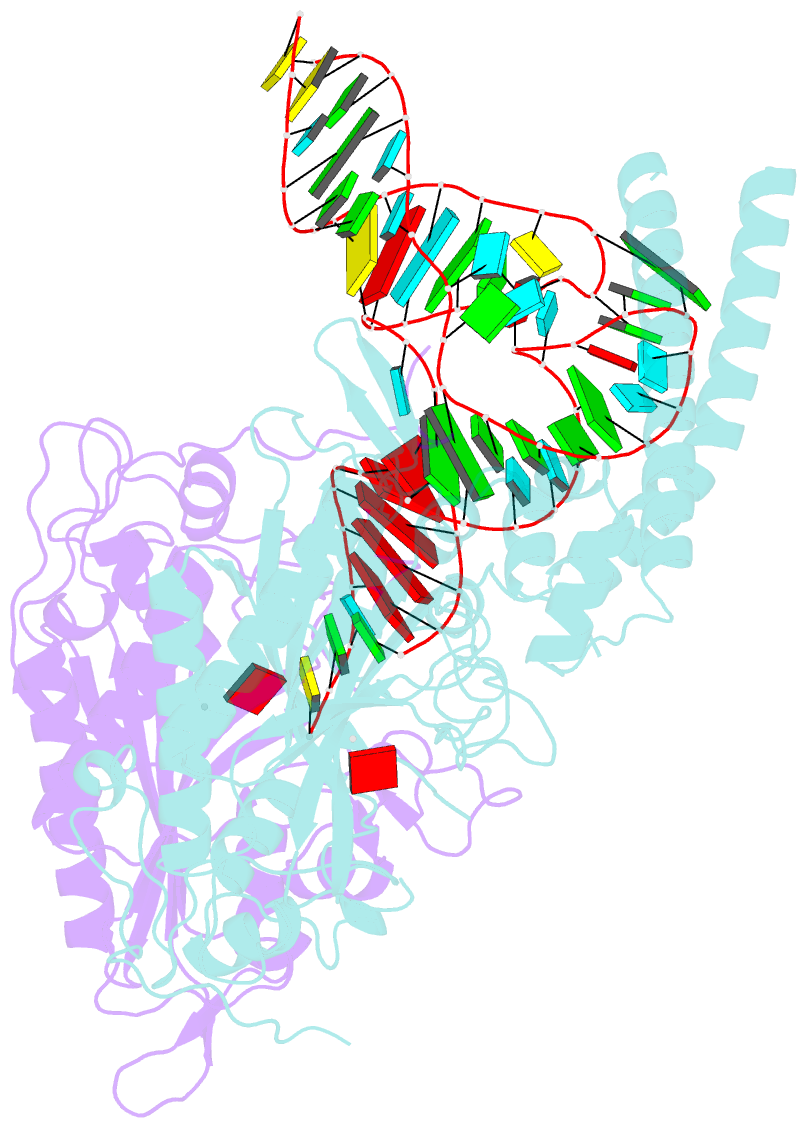
Summary information and primary citation
- PDB-id
- 8ffy; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ligase-RNA
- Method
- cryo-EM (3.6 Å)
- Summary
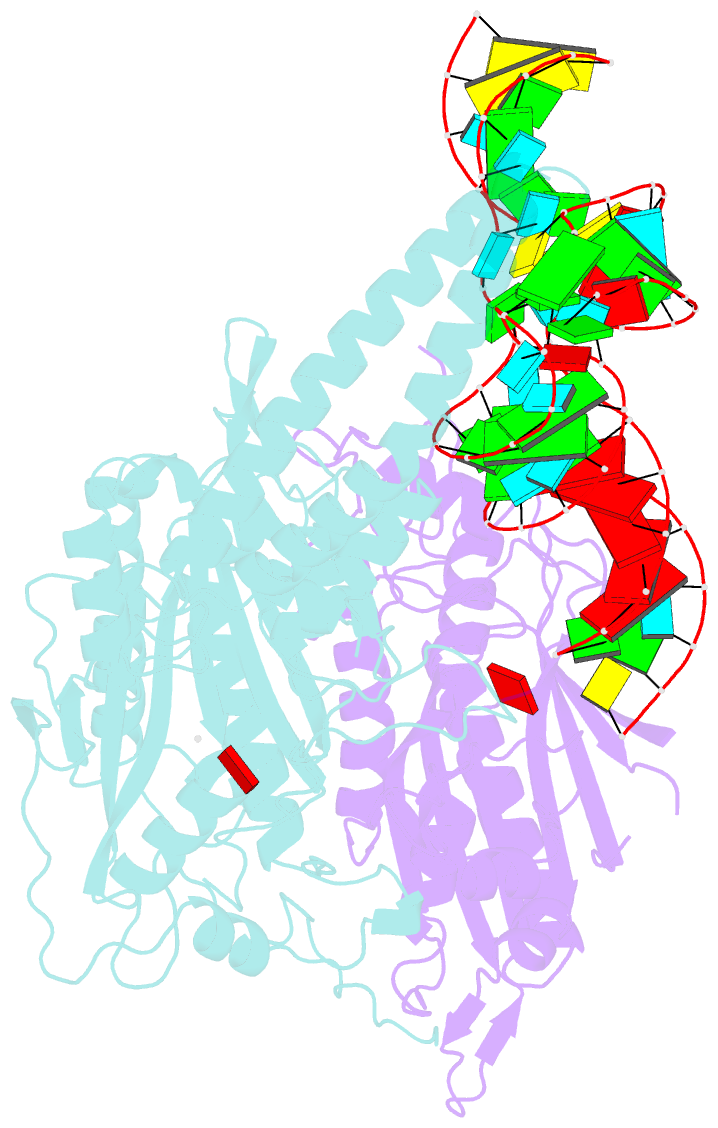
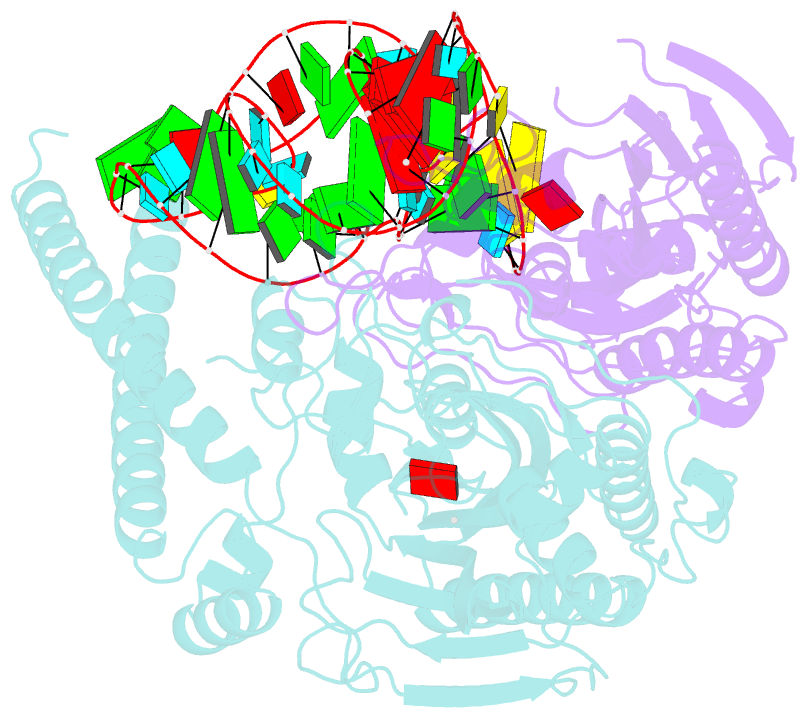
- Cryo-electron microscopy structure of human mt-serrs in complex with mt-trna(uga-tl)
- Reference
- Kuhle B, Hirschi M, Doerfel LK, Lander GC, Schimmel P (2023): "Structural basis for a degenerate tRNA identity code and the evolution of bimodal specificity in human mitochondrial tRNA recognition." Nat Commun, 14, 4794. doi: 10.1038/s41467-023-40354-2.
- Abstract
- Animal mitochondrial gene expression relies on specific interactions between nuclear-encoded aminoacyl-tRNA synthetases and mitochondria-encoded tRNAs. Their evolution involves an antagonistic interplay between strong mutation pressure on mtRNAs and selection pressure to maintain their essential function. To understand the molecular consequences of this interplay, we analyze the human mitochondrial serylation system, in which one synthetase charges two highly divergent mtRNASer isoacceptors. We present the cryo-EM structure of human mSerRS in complex with mtRNASer(UGA), and perform a structural and functional comparison with the mSerRS-mtRNASer(GCU) complex. We find that despite their common function, mtRNASer(UGA) and mtRNASer(GCU) show no constrain to converge on shared structural or sequence identity motifs for recognition by mSerRS. Instead, mSerRS evolved a bimodal readout mechanism, whereby a single protein surface recognizes degenerate identity features specific to each mtRNASer. Our results show how the mutational erosion of mtRNAs drove a remarkable innovation of intermolecular specificity rules, with multiple evolutionary pathways leading to functionally equivalent outcomes.