Summary information and primary citation
- PDB-id
- 8fim; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- X-ray (2.22 Å)
- Summary
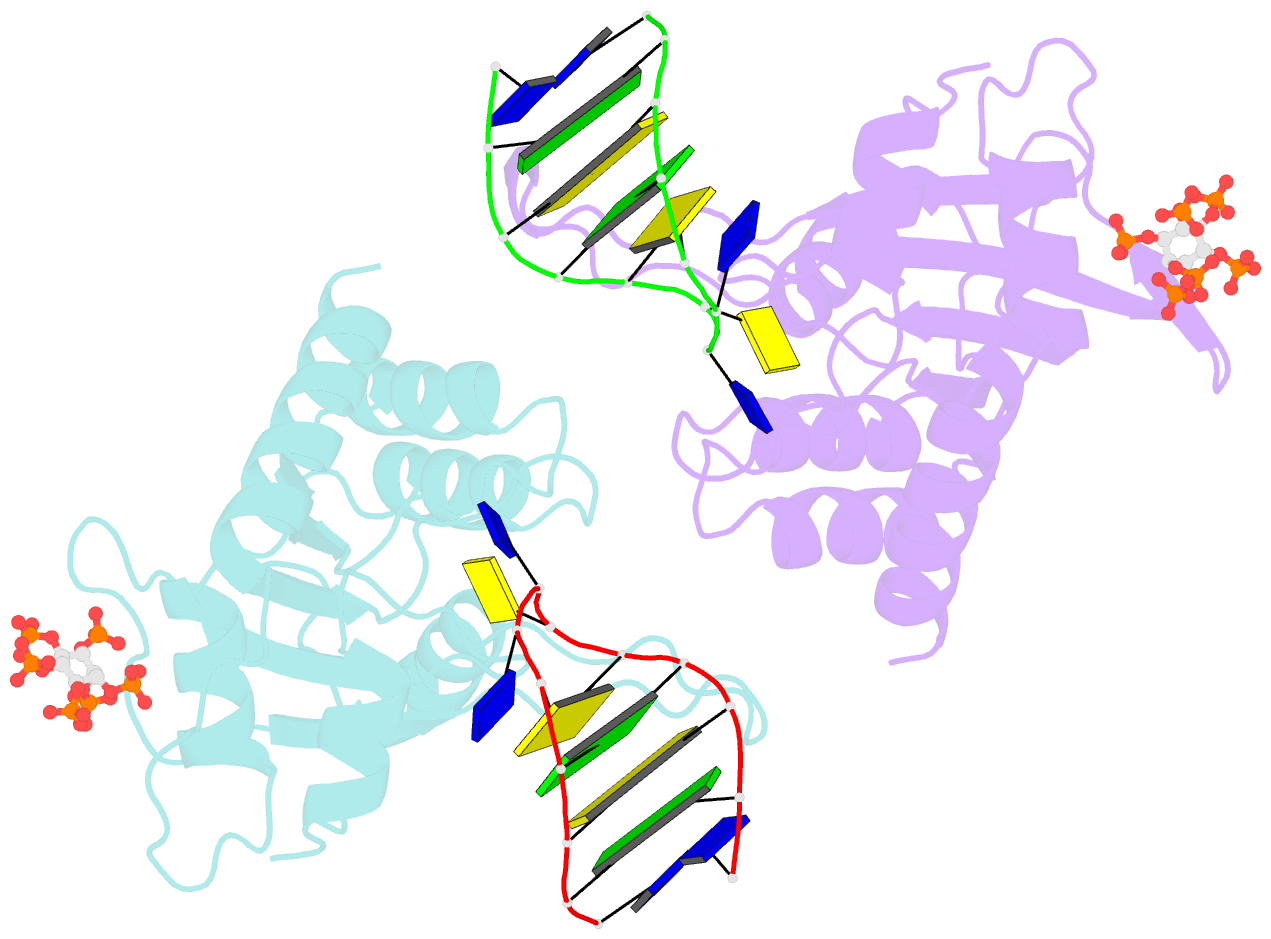
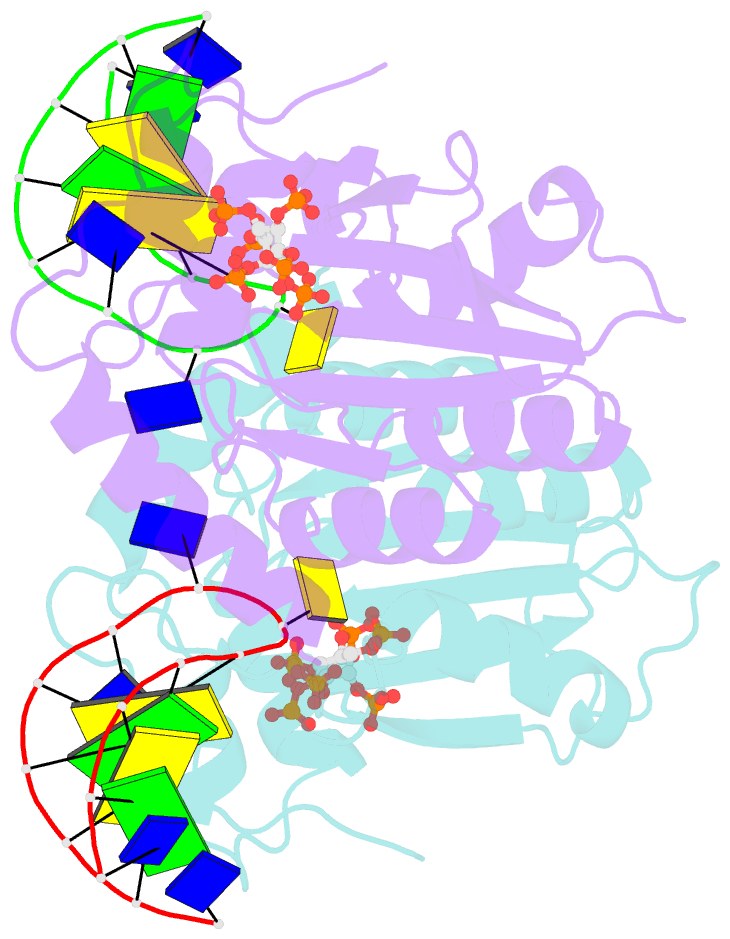
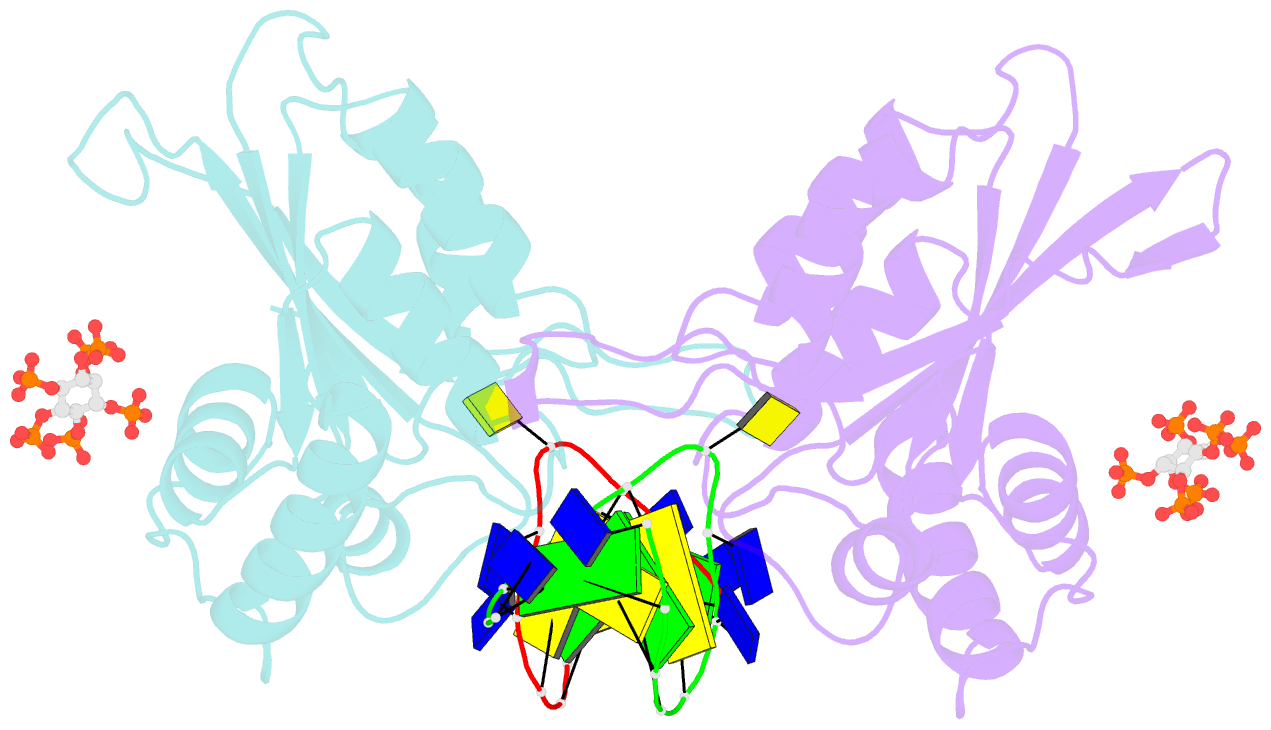
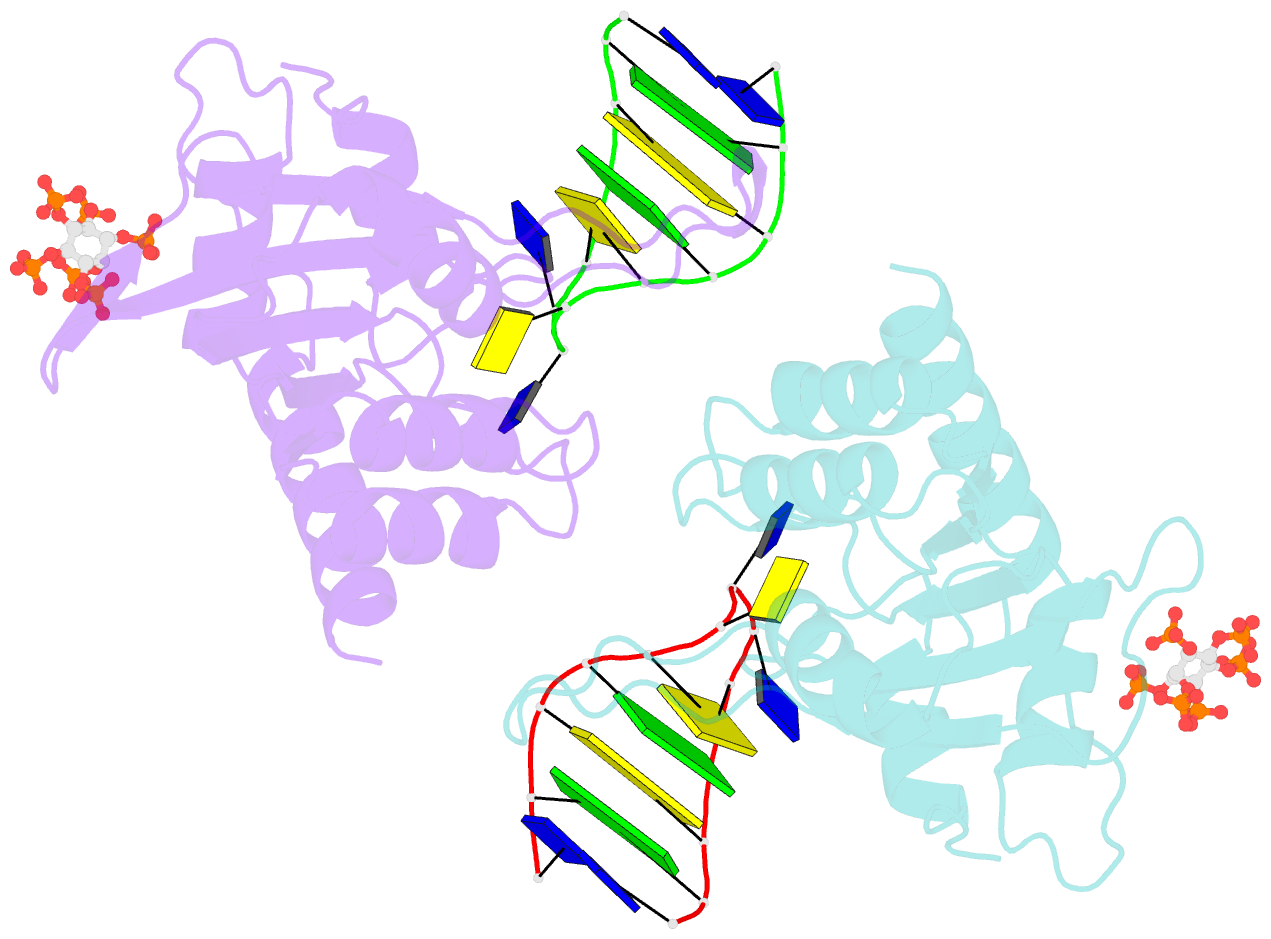
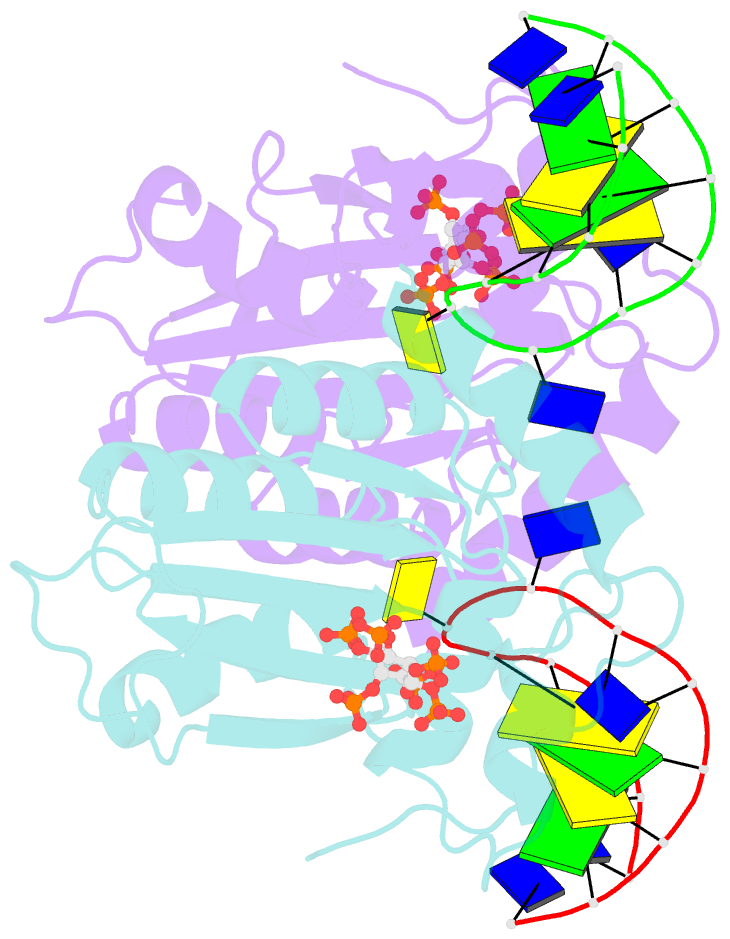
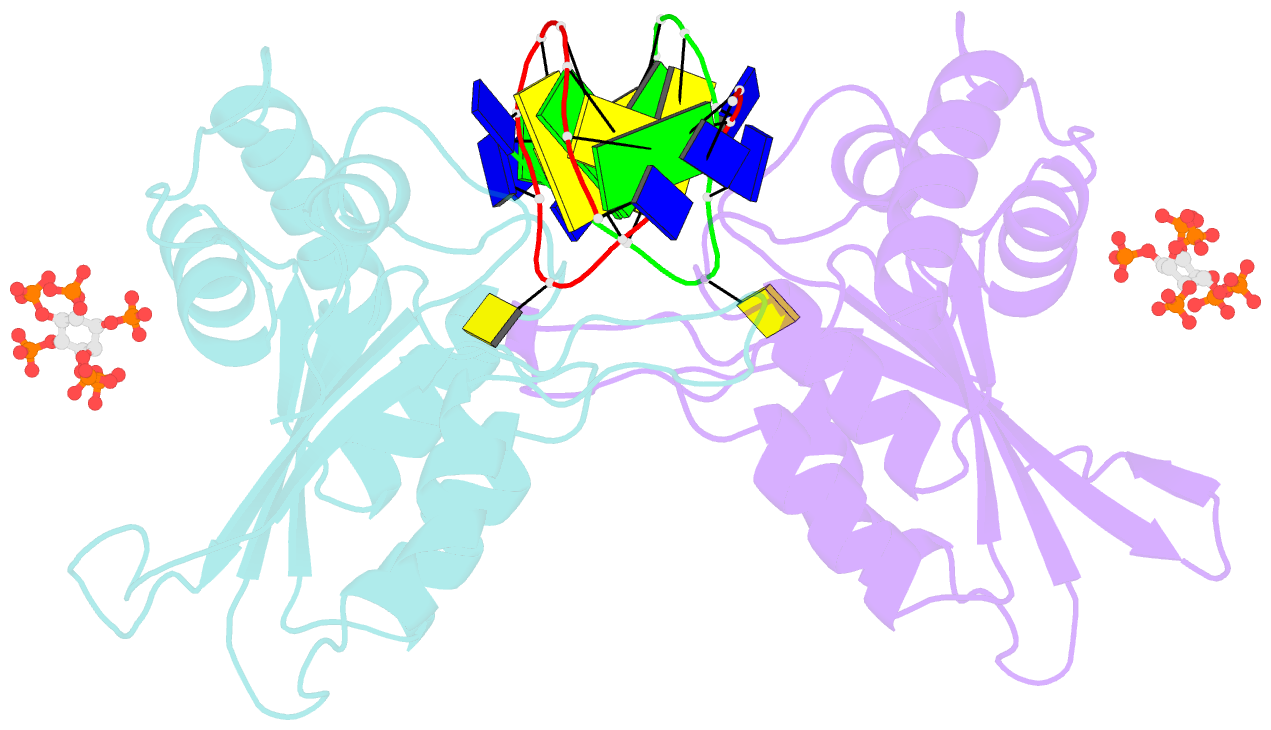
- Structure of apobec3a (e72a inactive mutant) in complex with ttc-hairpin DNA substrate
- Reference
- Harjes S, Kurup HM, Rieffer AE, Bayarjargal M, Filitcheva J, Su Y, Hale TK, Filichev VV, Harjes E, Harris RS, Jameson GB (2023): "Structure-guided inhibition of the cancer DNA-mutating enzyme APOBEC3A." Nat Commun, 14, 6382. doi: 10.1038/s41467-023-42174-w.
- Abstract
- The normally antiviral enzyme APOBEC3A is an endogenous mutagen in human cancer. Its single-stranded DNA C-to-U editing activity results in multiple mutagenic outcomes including signature single-base substitution mutations (isolated and clustered), DNA breakage, and larger-scale chromosomal aberrations. APOBEC3A inhibitors may therefore comprise a unique class of anti-cancer agents that work by blocking mutagenesis, slowing tumor evolvability, and preventing detrimental outcomes such as drug resistance and metastasis. Here we reveal the structural basis of competitive inhibition of wildtype APOBEC3A by hairpin DNA bearing 2'-deoxy-5-fluorozebularine in place of the cytidine in the TC substrate motif that is part of a 3-nucleotide loop. In addition, the structural basis of APOBEC3A's preference for YTCD motifs (Y = T, C; D = A, G, T) is explained. The nuclease-resistant phosphorothioated derivatives of these inhibitors have nanomolar potency in vitro and block APOBEC3A activity in human cells. These inhibitors may be useful probes for studying APOBEC3A activity in cellular systems and leading toward, potentially as conjuvants, next-generation, combinatorial anti-mutator and anti-cancer therapies.