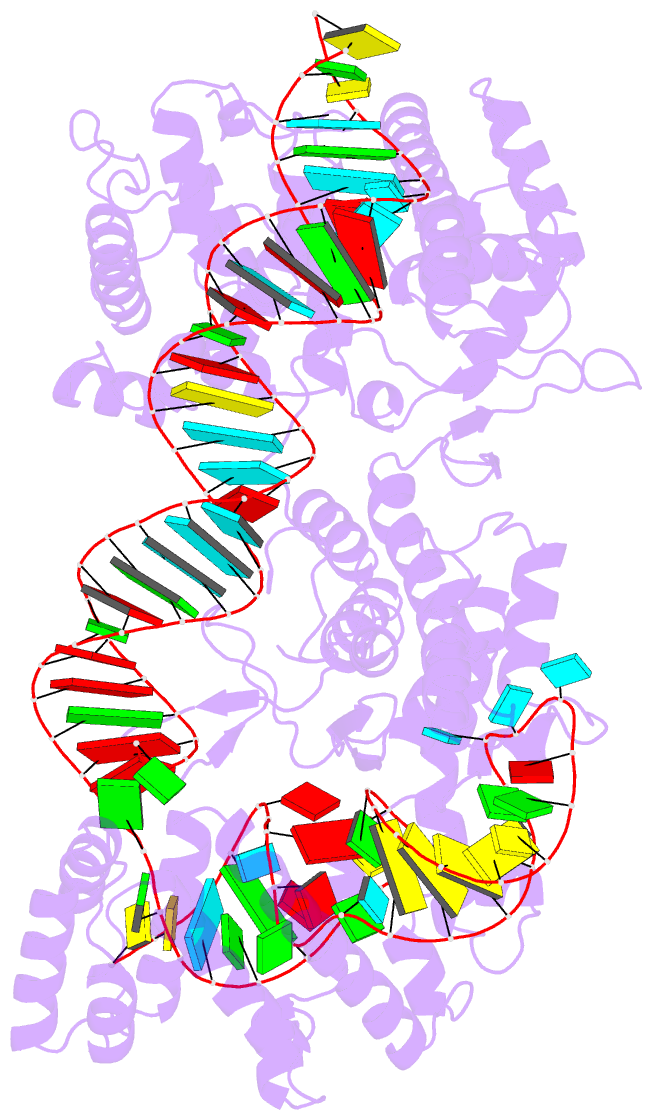
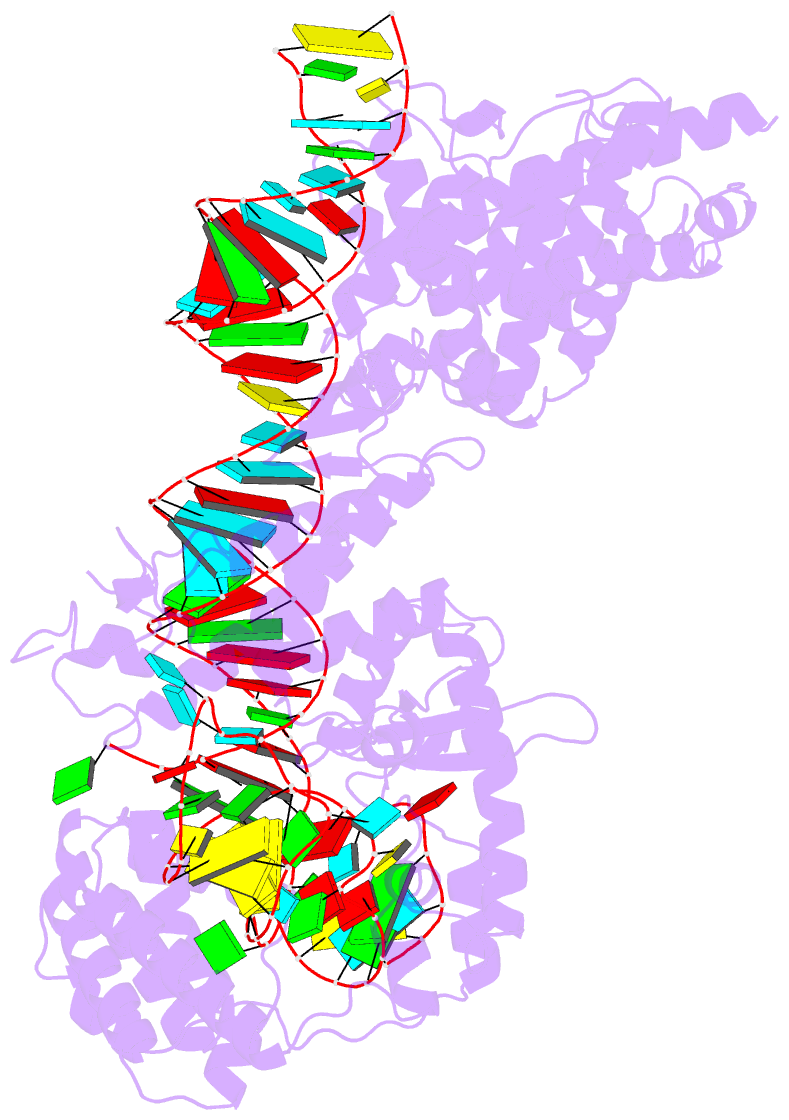
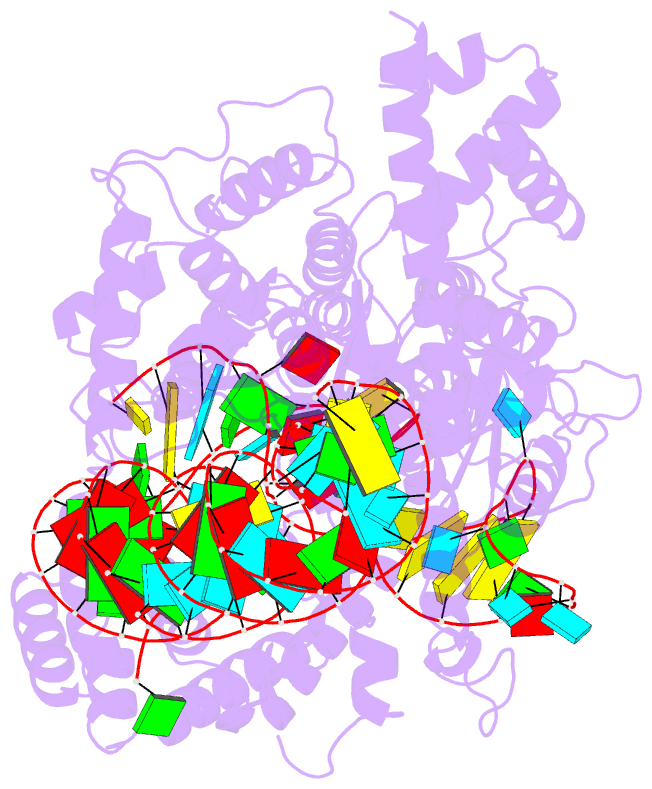
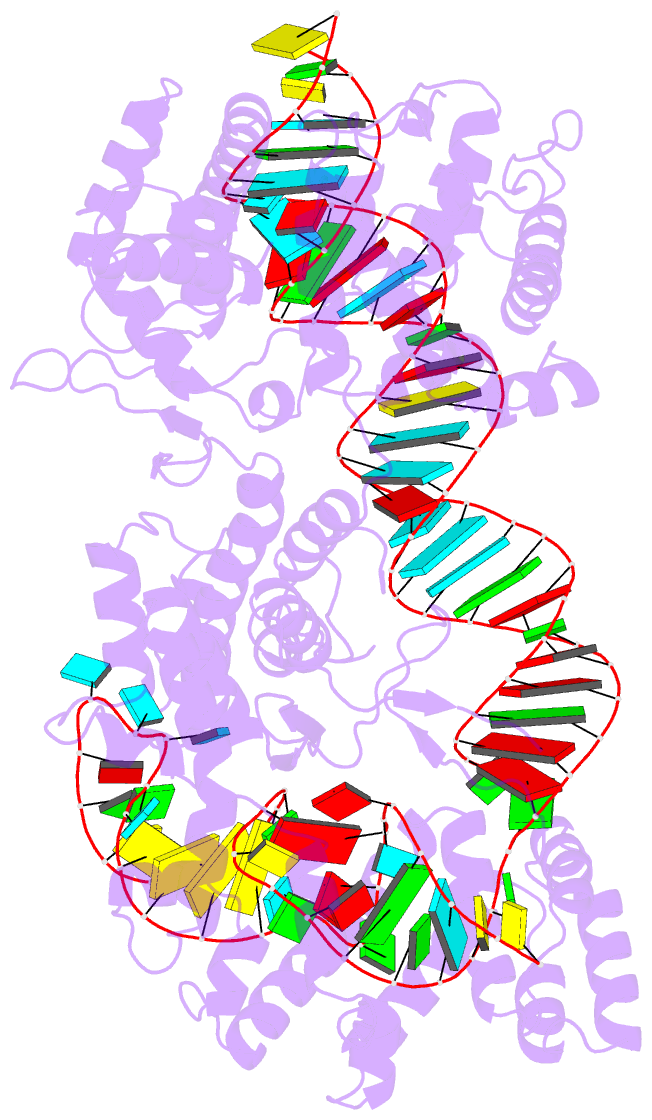
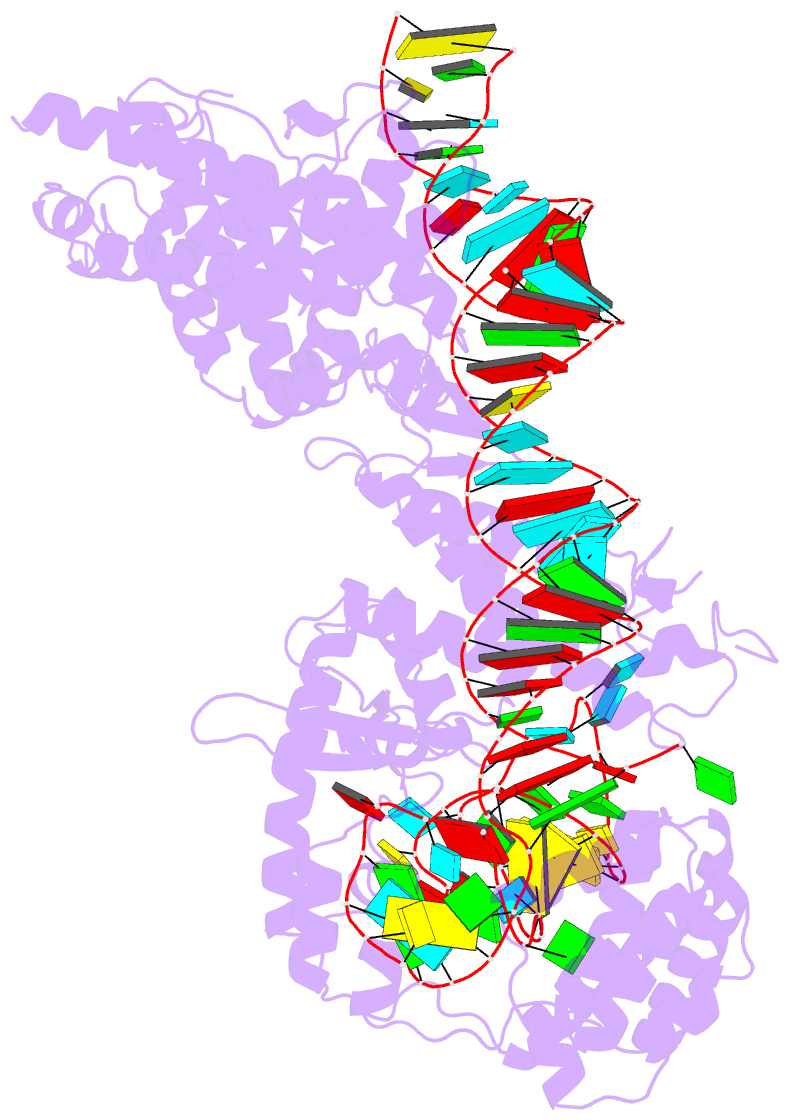
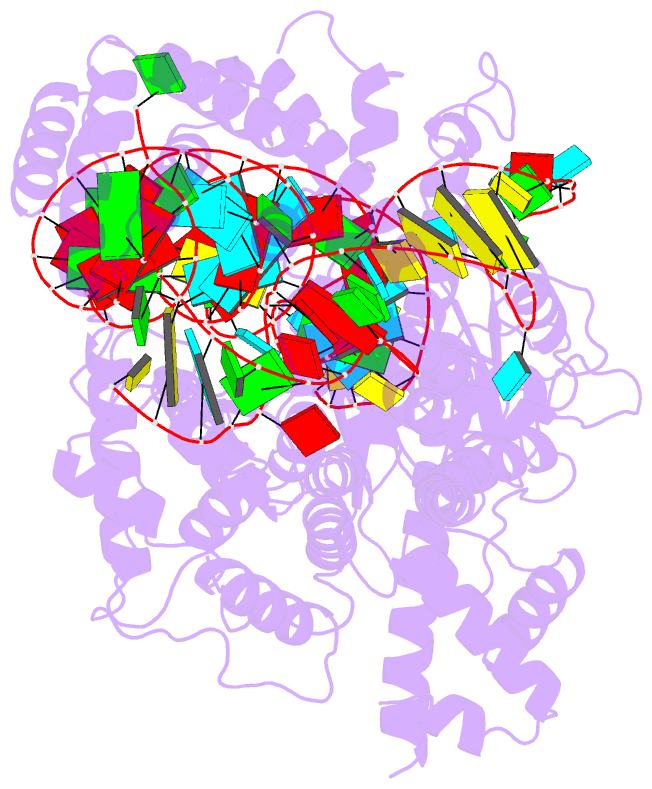
Summary information and primary citation
- PDB-id
- 8fti; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- cryo-EM (3.5 Å)
- Summary
- cryo-EM structure of the cas13bt3-crrna-target RNA ternary complex in activated state
- Reference
- Deng X, Osikpa E, Yang J, Oladeji SJ, Smith J, Gao X, Gao Y (2023): "Structural basis for the activation of a compact CRISPR-Cas13 nuclease." Nat Commun, 14, 5845. doi: 10.1038/s41467-023-41501-5.
- Abstract
- The CRISPR-Cas13 ribonucleases have been widely applied for RNA knockdown and transcriptional modulation owing to their high programmability and specificity. However, the large size of Cas13 effectors and their non-specific RNA cleavage upon target activation limit the adeno-associated virus based delivery of Cas13 systems for therapeutic applications. Herein, we report detailed biochemical and structural characterizations of a compact Cas13 (Cas13bt3) suitable for adeno-associated virus delivery. Distinct from many other Cas13 systems, Cas13bt3 cleaves the target and other nonspecific RNA at internal "UC" sites and is activated in a target length-dependent manner. The cryo-electron microscope structure of Cas13bt3 in a fully active state illustrates the structural basis of Cas13bt3 activation. Guided by the structure, we obtain engineered Cas13bt3 variants with minimal off-target cleavage yet maintained target cleavage activities. In conclusion, our biochemical and structural data illustrate a distinct mechanism for Cas13bt3 activation and guide the engineering of Cas13bt3 applications.