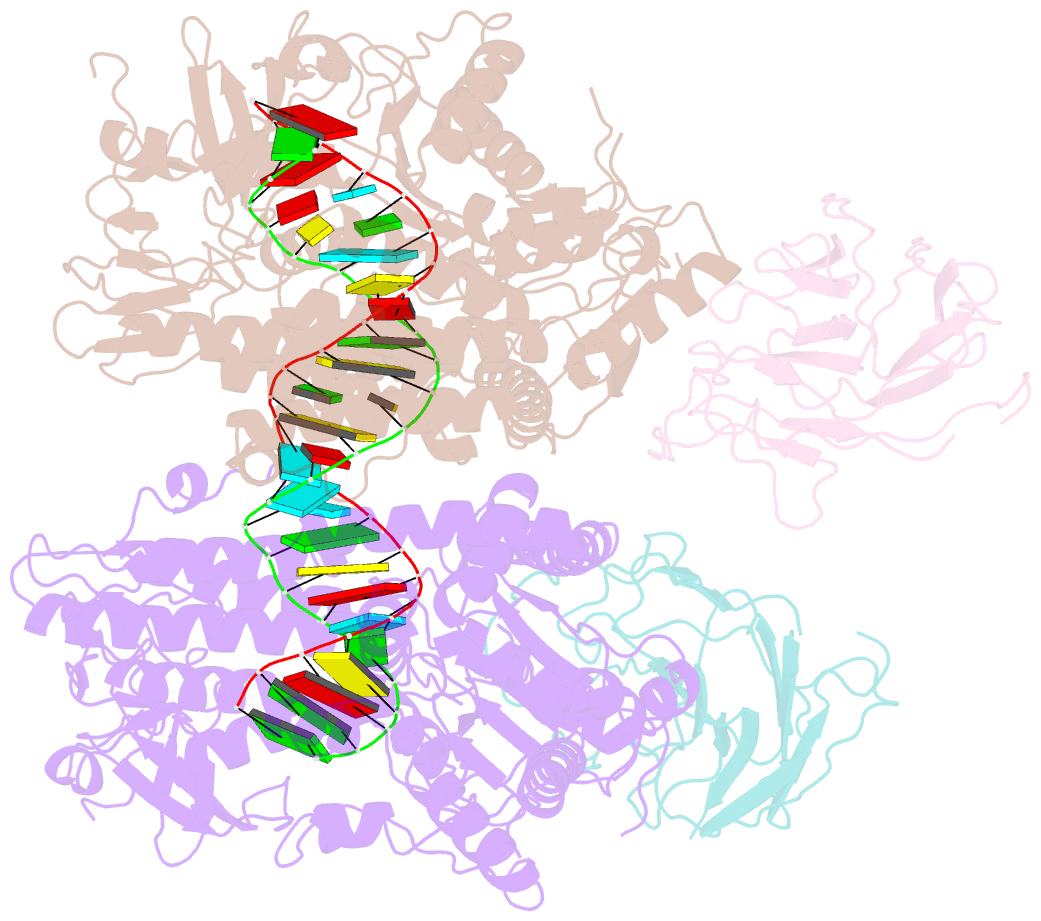
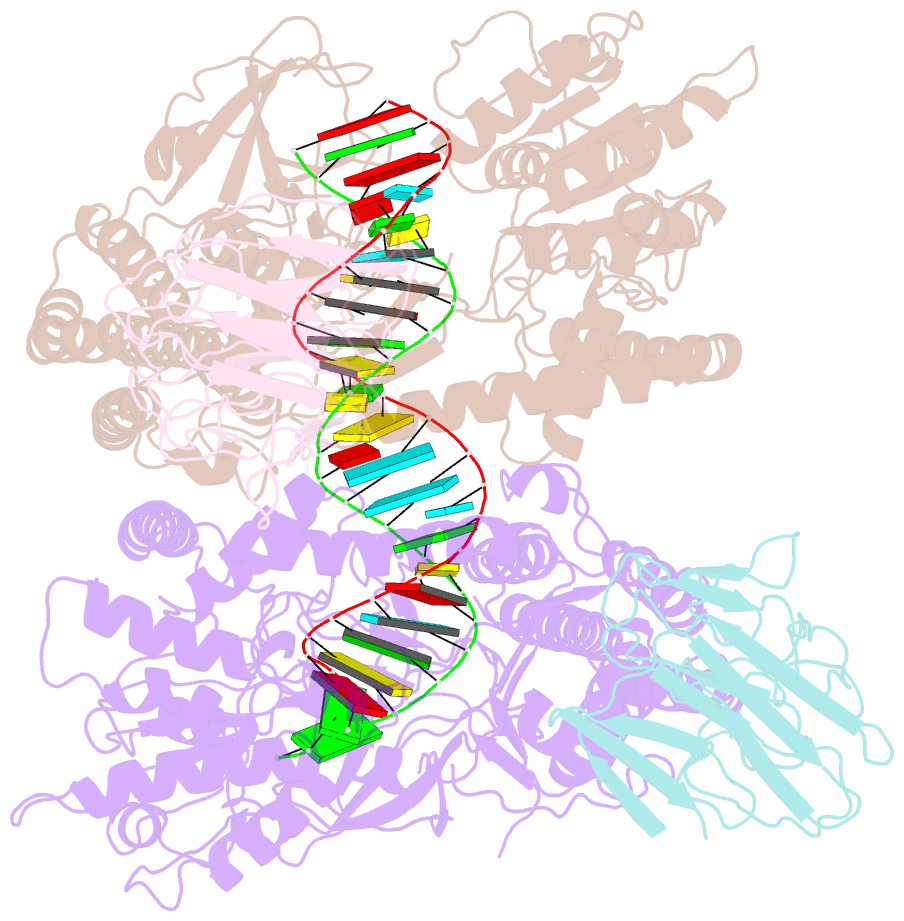
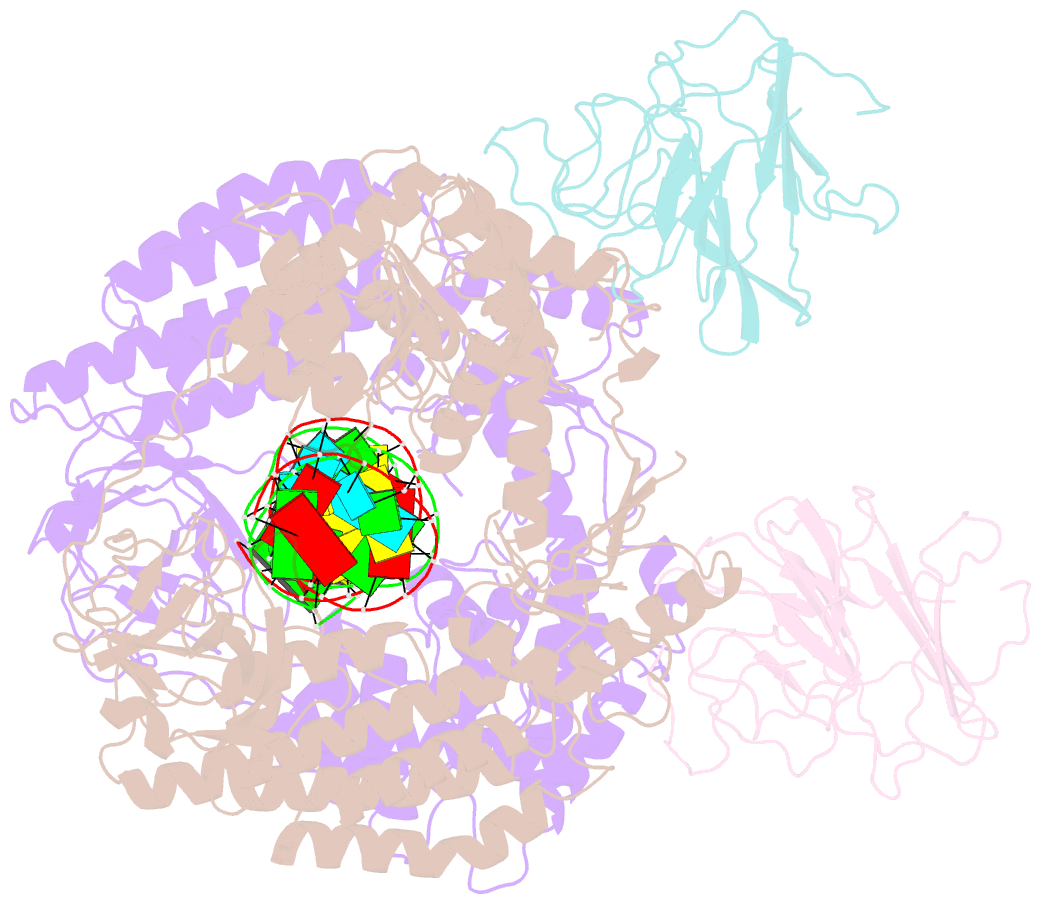
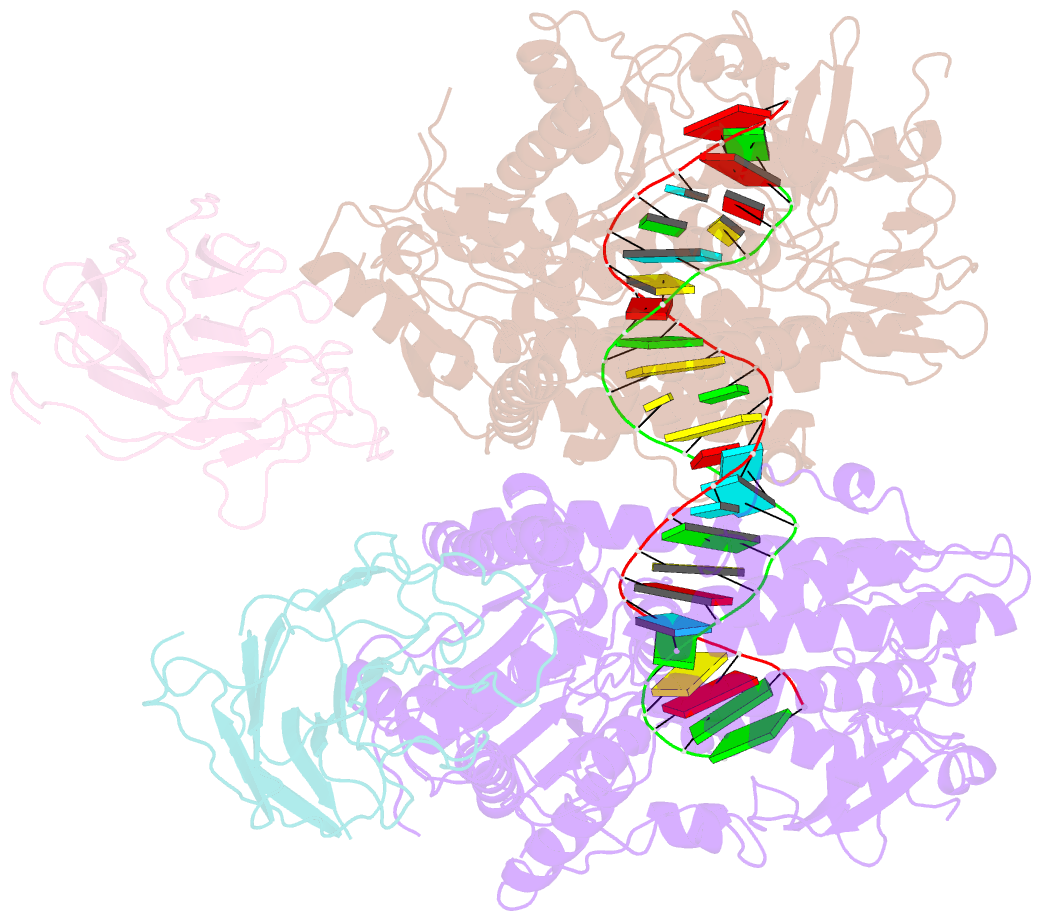
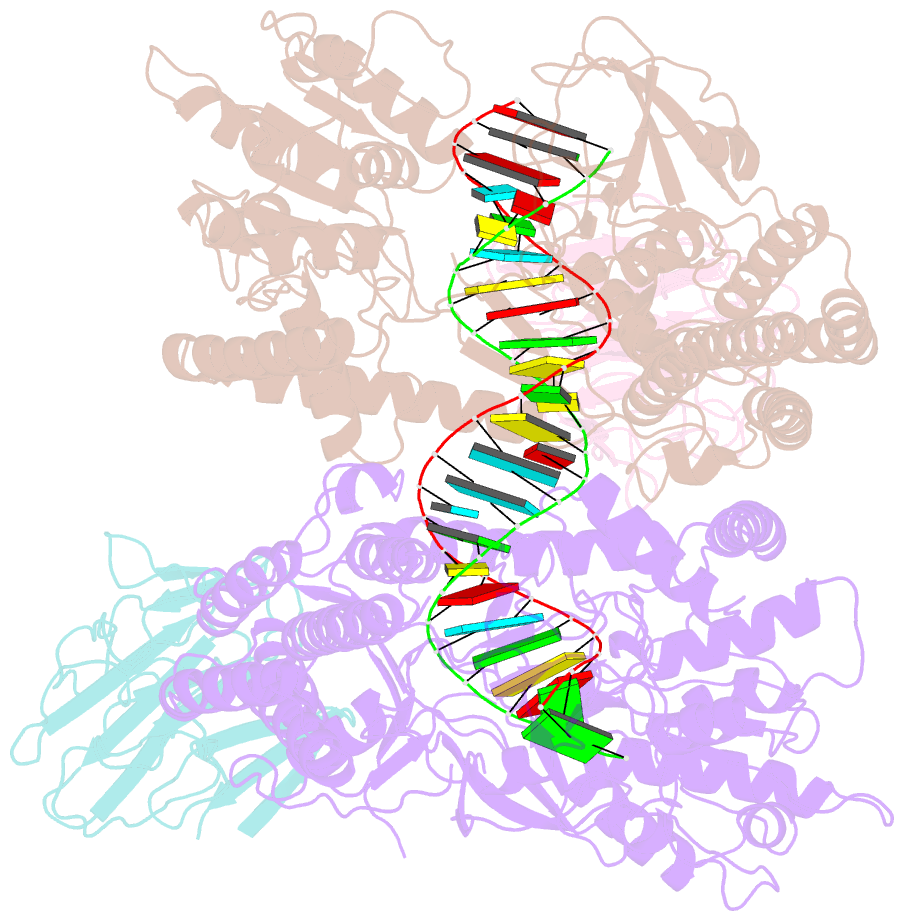
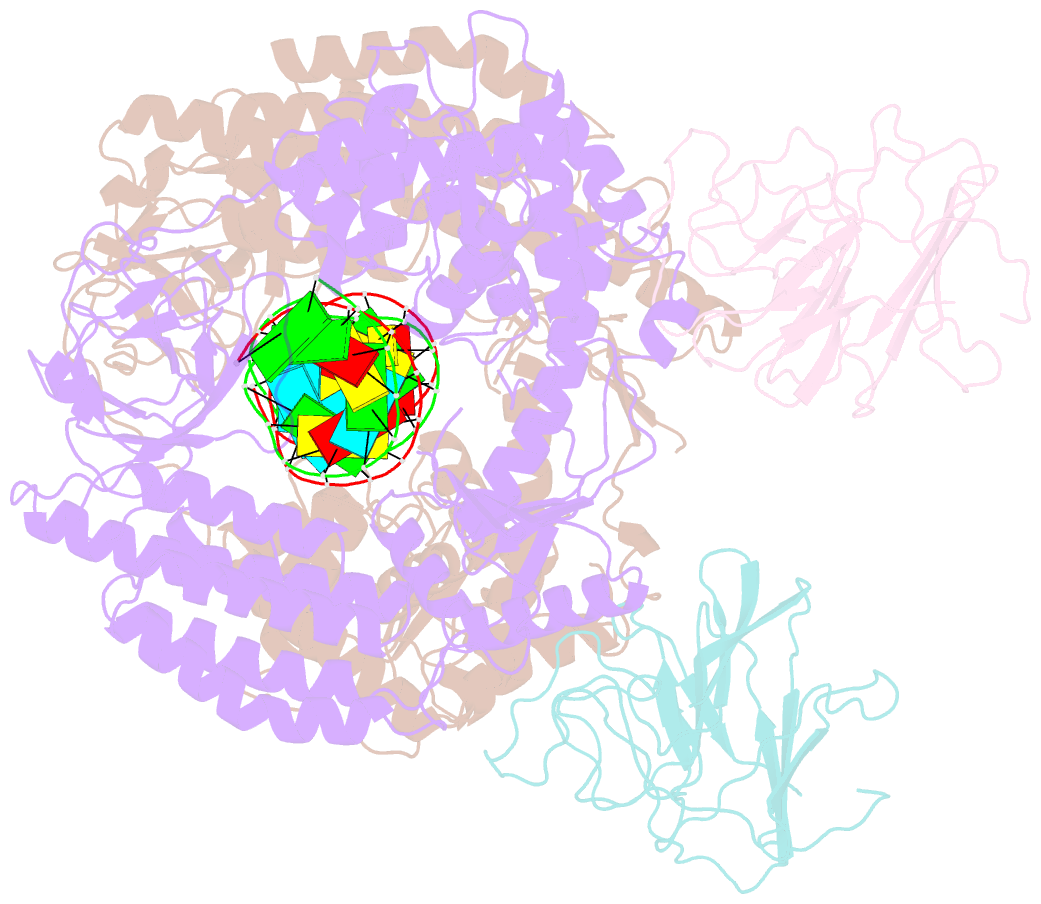
Summary information and primary citation
- PDB-id
- 8g7u; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-hydrolase-RNA
- Method
- cryo-EM (4.0 Å)
- Summary
- cryo-EM structure of riplet:rig-i:dsrna complex (end-semi-closed end)
- Reference
- Wang W, Gotte B, Guo R, Pyle AM (2023): "The E3 ligase Riplet promotes RIG-I signaling independent of RIG-I oligomerization." Nat Commun, 14, 7308. doi: 10.1038/s41467-023-42982-0.
- Abstract
- RIG-I is an essential innate immune receptor that responds to infection by RNA viruses. The RIG-I signaling cascade is mediated by a series of post-translational modifications, the most important of which is ubiquitination of the RIG-I Caspase Recruitment Domains (CARDs) by E3 ligase Riplet. This is required for interaction between RIG-I and its downstream adapter protein MAVS, but the mechanism of action remains unclear. Here we show that Riplet is required for RIG-I signaling in the presence of both short and long dsRNAs, establishing that Riplet activation does not depend upon RIG-I filament formation on long dsRNAs. Likewise, quantitative Riplet-RIG-I affinity measurements establish that Riplet interacts with RIG-I regardless of whether the receptor is bound to RNA. To understand this, we solved high-resolution cryo-EM structures of RIG-I/RNA/Riplet complexes, revealing molecular interfaces that control Riplet-mediated activation and enabling the formulation of a unified model for the role of Riplet in signaling.