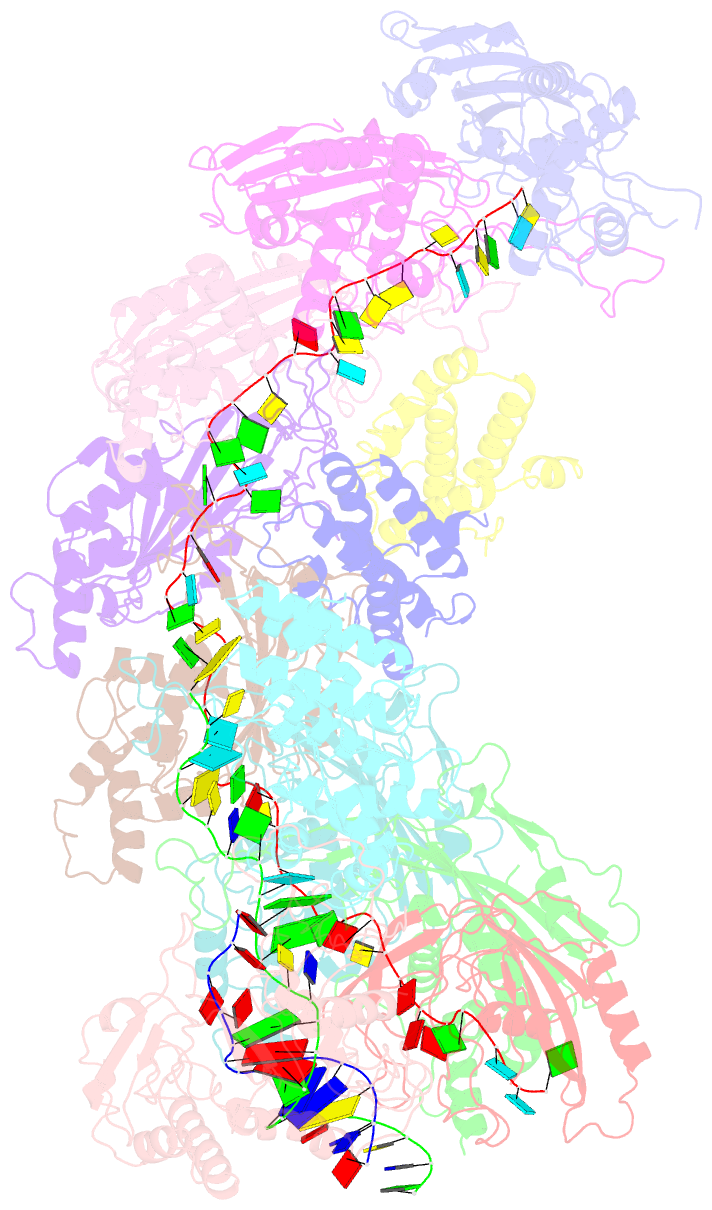
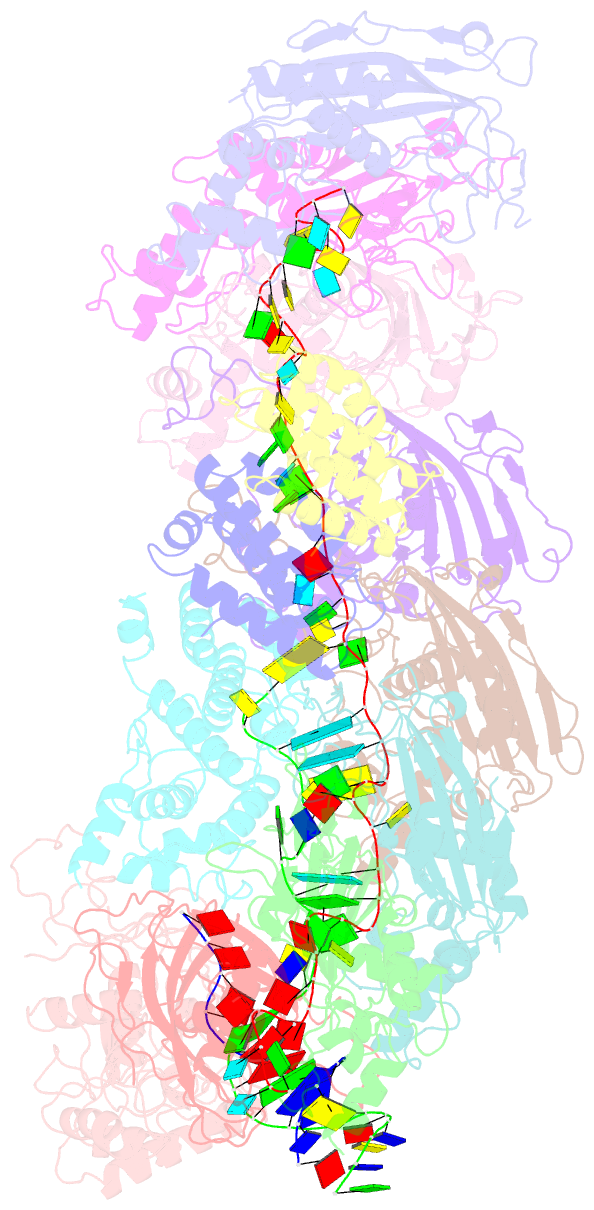
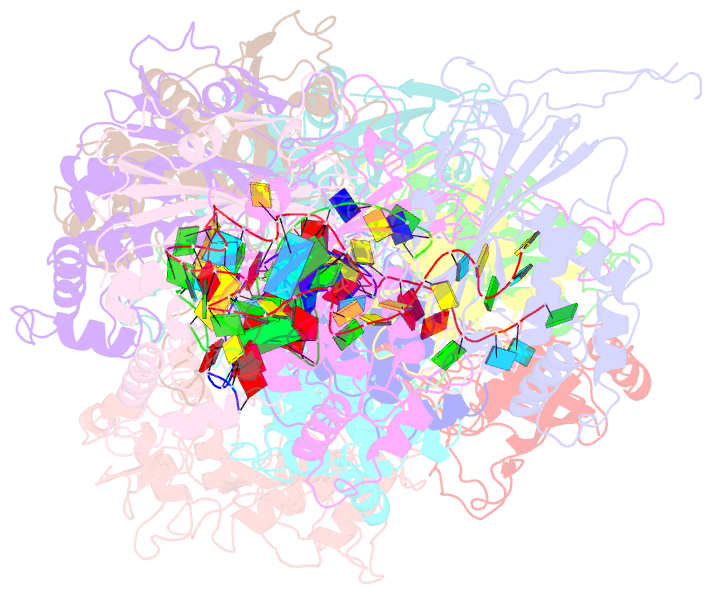
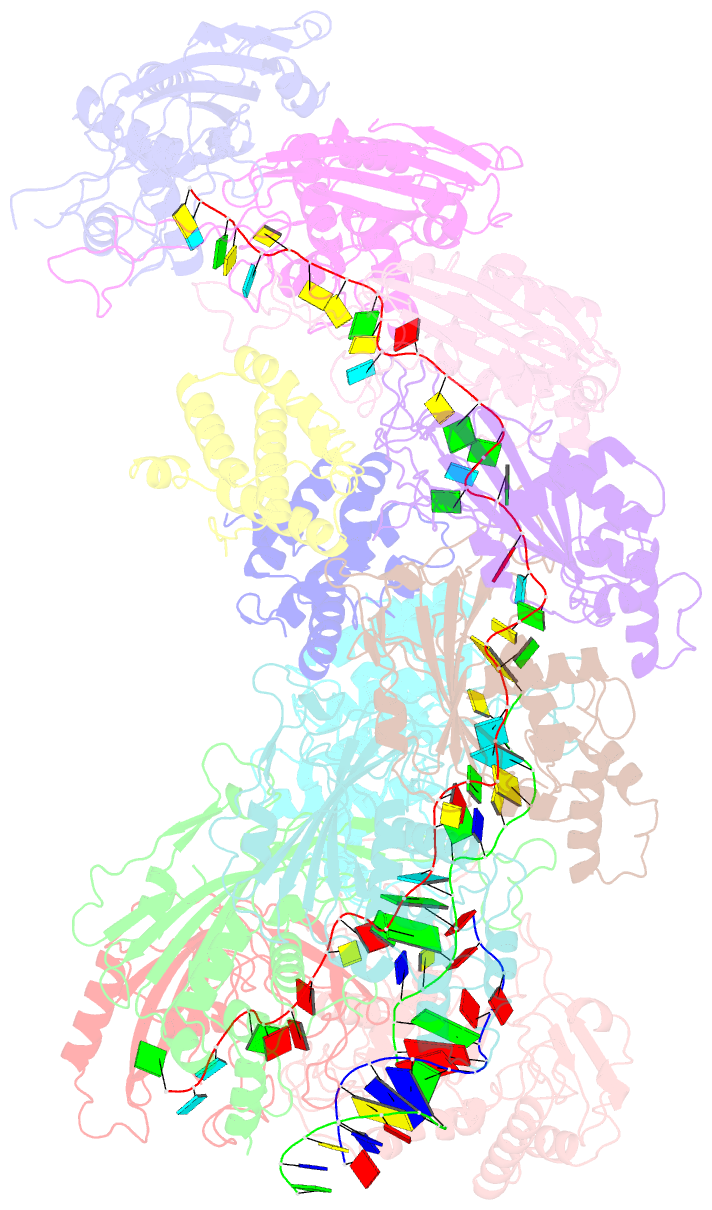
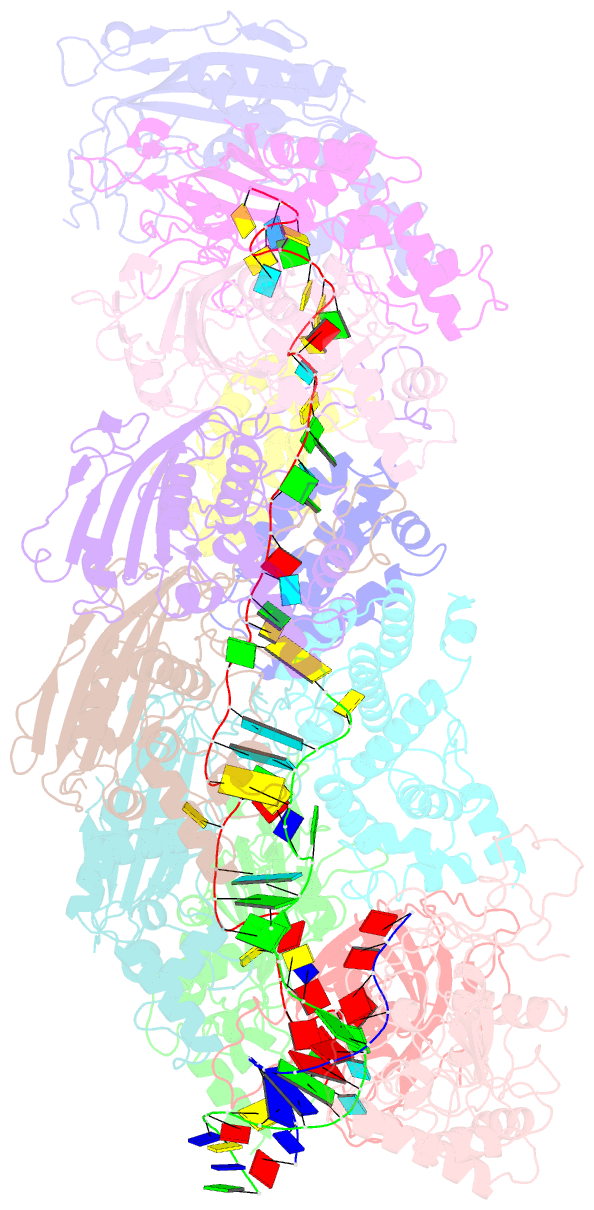
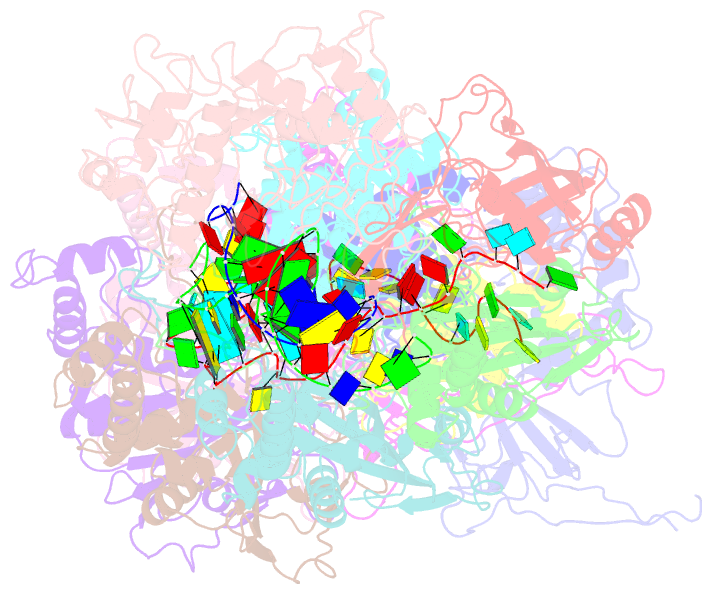
Summary information and primary citation
- PDB-id
- 8gam; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-RNA-DNA
- Method
- cryo-EM (3.46 Å)
- Summary
- Exploiting activation and inactivation mechanisms in type i-c crispr-cas3 for genome editing applications
- Reference
- Hu C, Myers MT, Zhou X, Hou Z, Lozen ML, Nam KH, Zhang Y, Ke A (2024): "Exploiting activation and inactivation mechanisms in type I-C CRISPR-Cas3 for genome-editing applications." Mol.Cell, 84, 463-475.e5. doi: 10.1016/j.molcel.2023.12.034.
- Abstract
- Type I CRISPR-Cas systems utilize the RNA-guided Cascade complex to identify matching DNA targets and the nuclease-helicase Cas3 to degrade them. Among the seven subtypes, type I-C is compact in size and highly active in creating large-sized genome deletions in human cells. Here, we use four cryoelectron microscopy snapshots to define its RNA-guided DNA binding and cleavage mechanisms in high resolution. The non-target DNA strand (NTS) is accommodated by I-C Cascade in a continuous binding groove along the juxtaposed Cas11 subunits. Binding of Cas3 further traps a flexible bulge in NTS, enabling NTS nicking. We identified two anti-CRISPR proteins AcrIC8 and AcrIC9 that strongly inhibit Neisseria lactamica I-C function. Structural analysis showed that AcrIC8 inhibits PAM recognition through allosteric inhibition, whereas AcrIC9 achieves so through direct competition. Both Acrs potently inhibit I-C-mediated genome editing and transcriptional modulation in human cells, providing the first off-switches for type I CRISPR eukaryotic genome engineering.