Summary information and primary citation
- PDB-id
- 8gmu; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- cryo-EM (2.78 Å)
- Summary
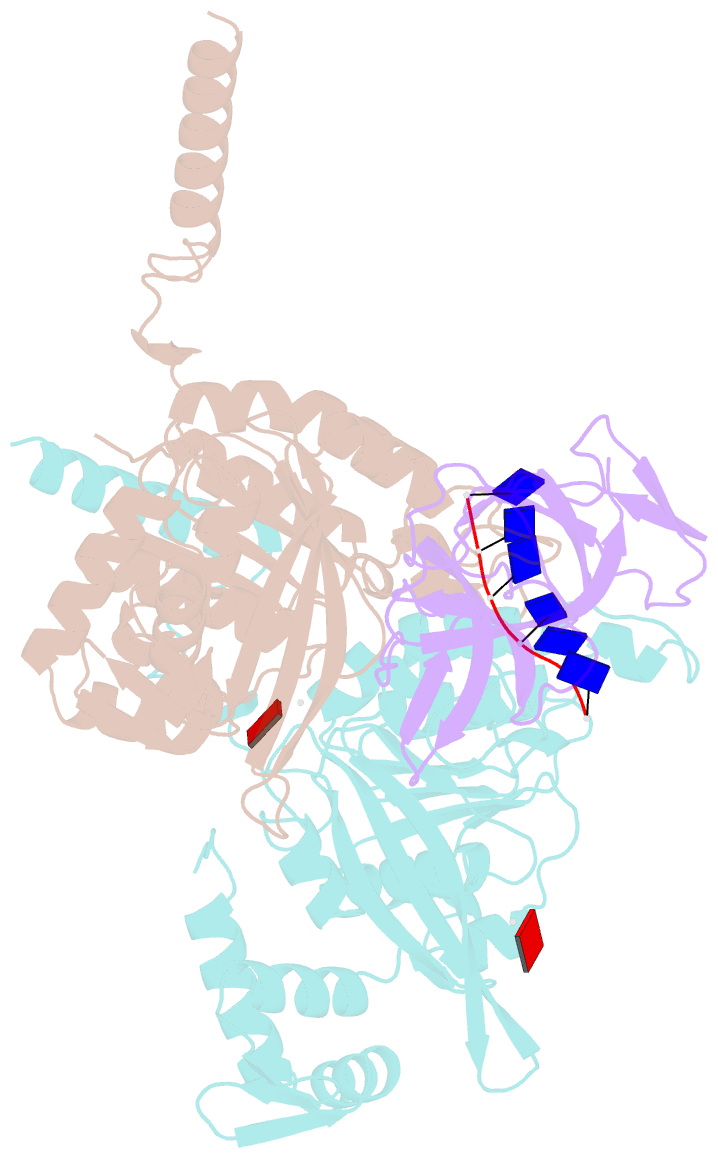
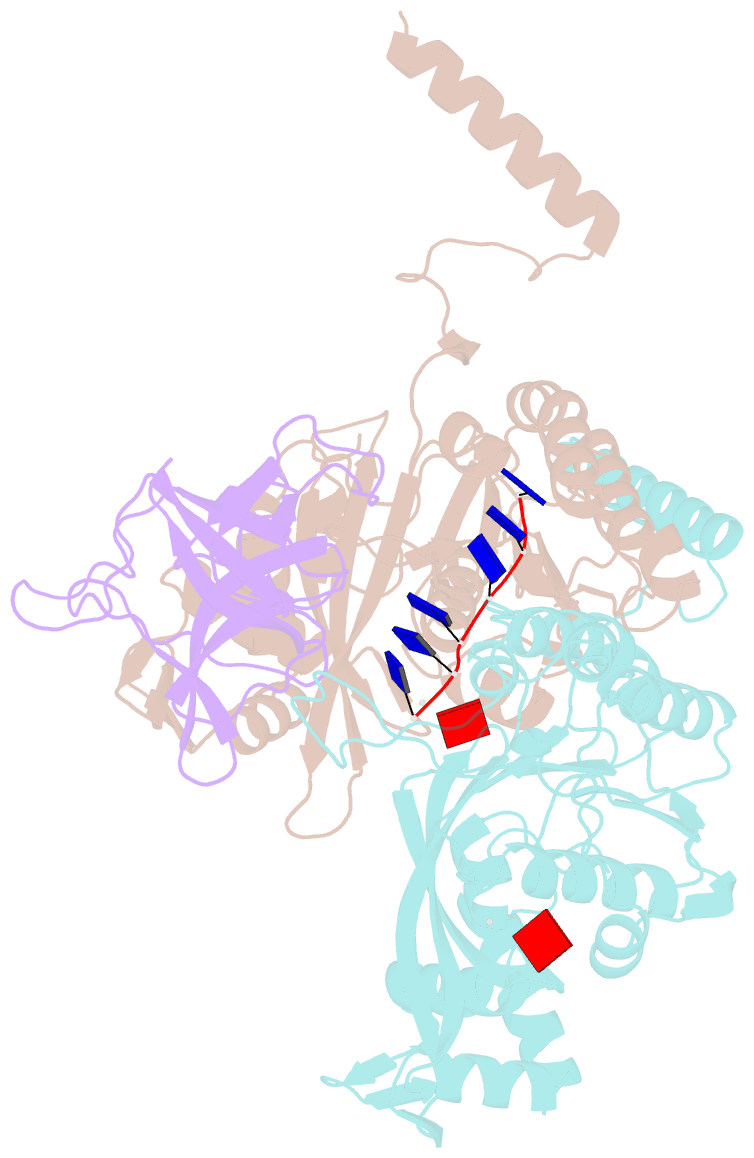
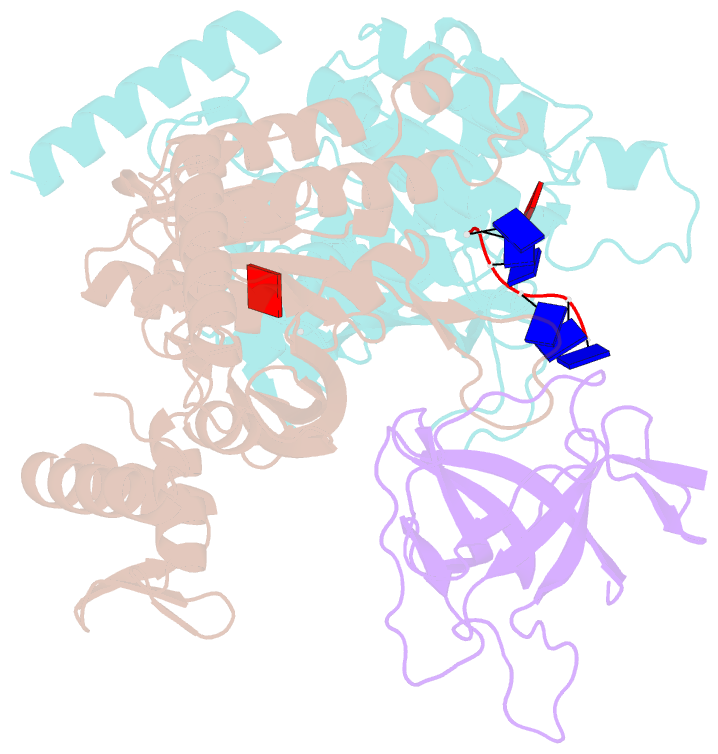
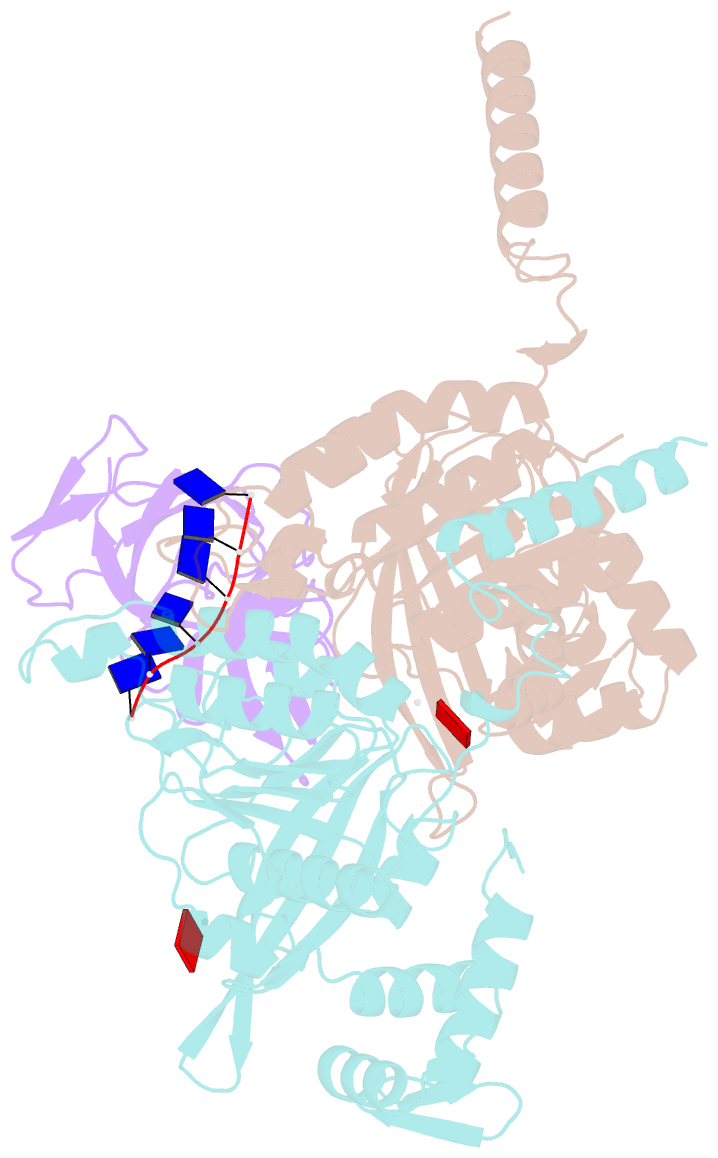
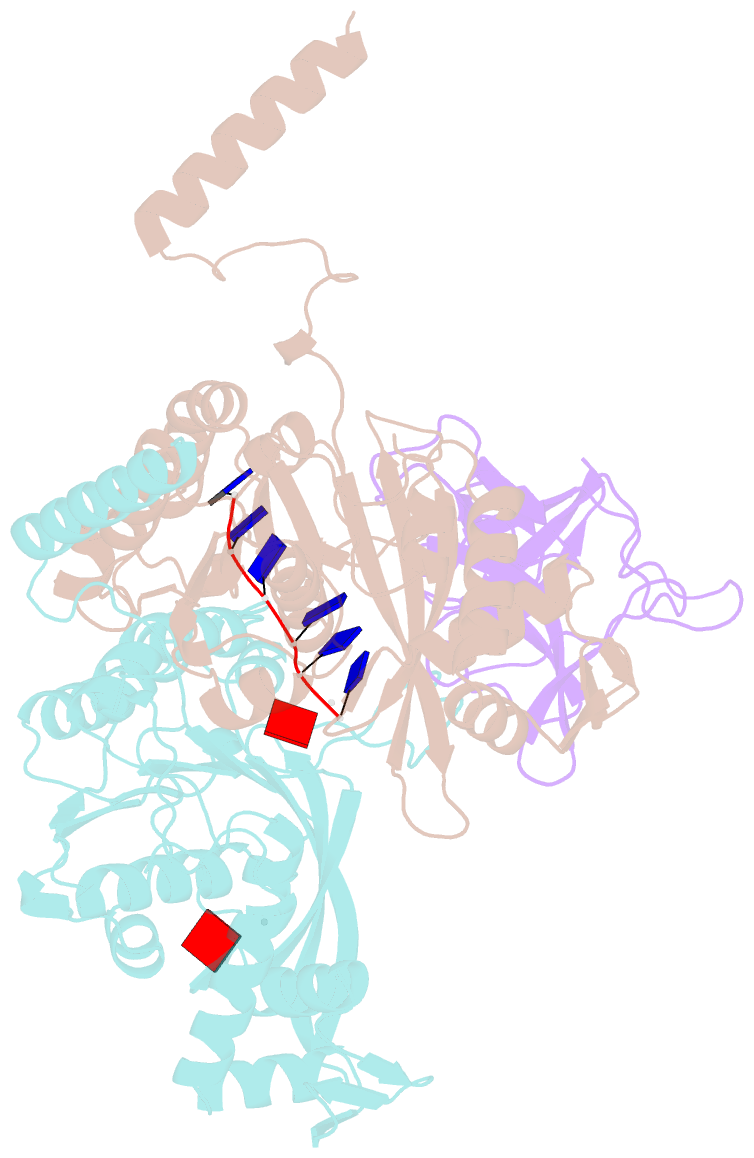
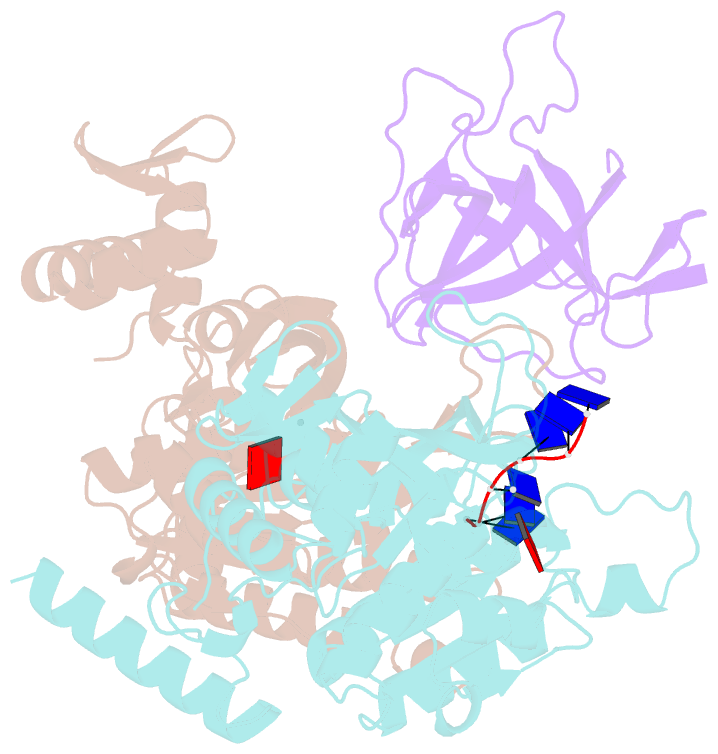
- Structure of lambda repressor in complex with reca filament
- Reference
- Gao B, Liang L, Su L, Wen A, Zhou C, Feng Y (2023): "Structural basis for regulation of SOS response in bacteria." Proc.Natl.Acad.Sci.USA, 120, e2217493120. doi: 10.1073/pnas.2217493120.
- Abstract
- In response to DNA damage, bacterial RecA protein forms filaments with the assistance of DinI protein. The RecA filaments stimulate the autocleavage of LexA, the repressor of more than 50 SOS genes, and activate the SOS response. During the late phase of SOS response, the RecA filaments stimulate the autocleavage of UmuD and λ repressor CI, leading to mutagenic repair and lytic cycle, respectively. Here, we determined the cryo-electron microscopy structures of Escherichia coli RecA filaments in complex with DinI, LexA, UmuD, and λCI by helical reconstruction. The structures reveal that LexA and UmuD dimers bind in the filament groove and cleave in an intramolecular and an intermolecular manner, respectively, while λCI binds deeply in the filament groove as a monomer. Despite their distinct folds and oligomeric states, all RecA filament binders recognize the same conserved protein features in the filament groove. The SOS response in bacteria can lead to mutagenesis and antimicrobial resistance, and our study paves the way for rational drug design targeting the bacterial SOS response.