Summary information and primary citation
- PDB-id
- 8guj; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- gene regulation-DNA
- Method
- cryo-EM (2.8 Å)
- Summary
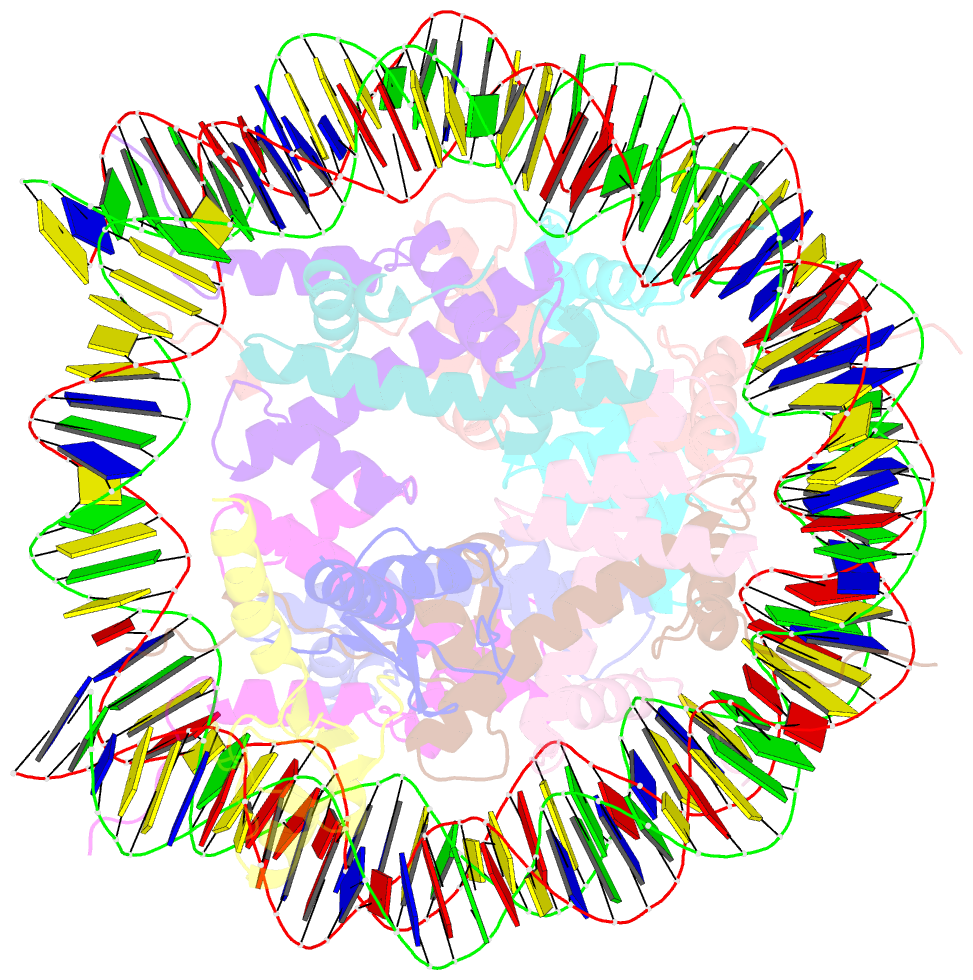
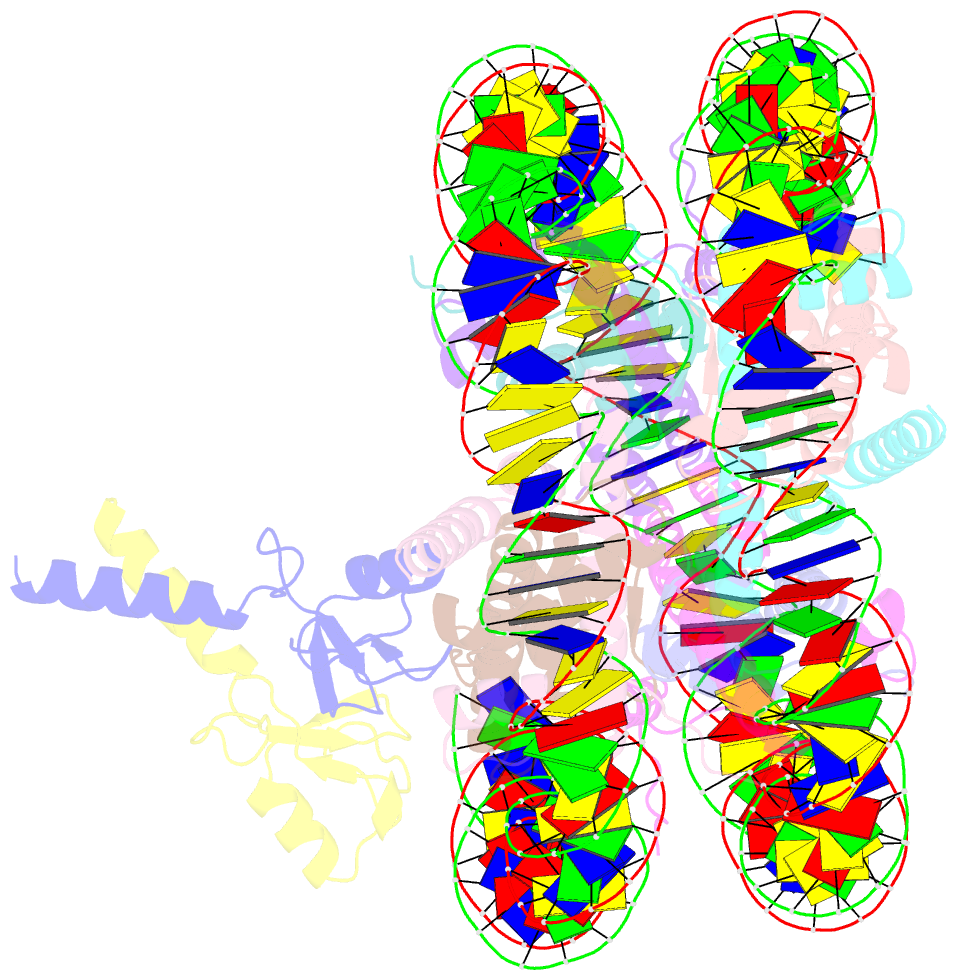
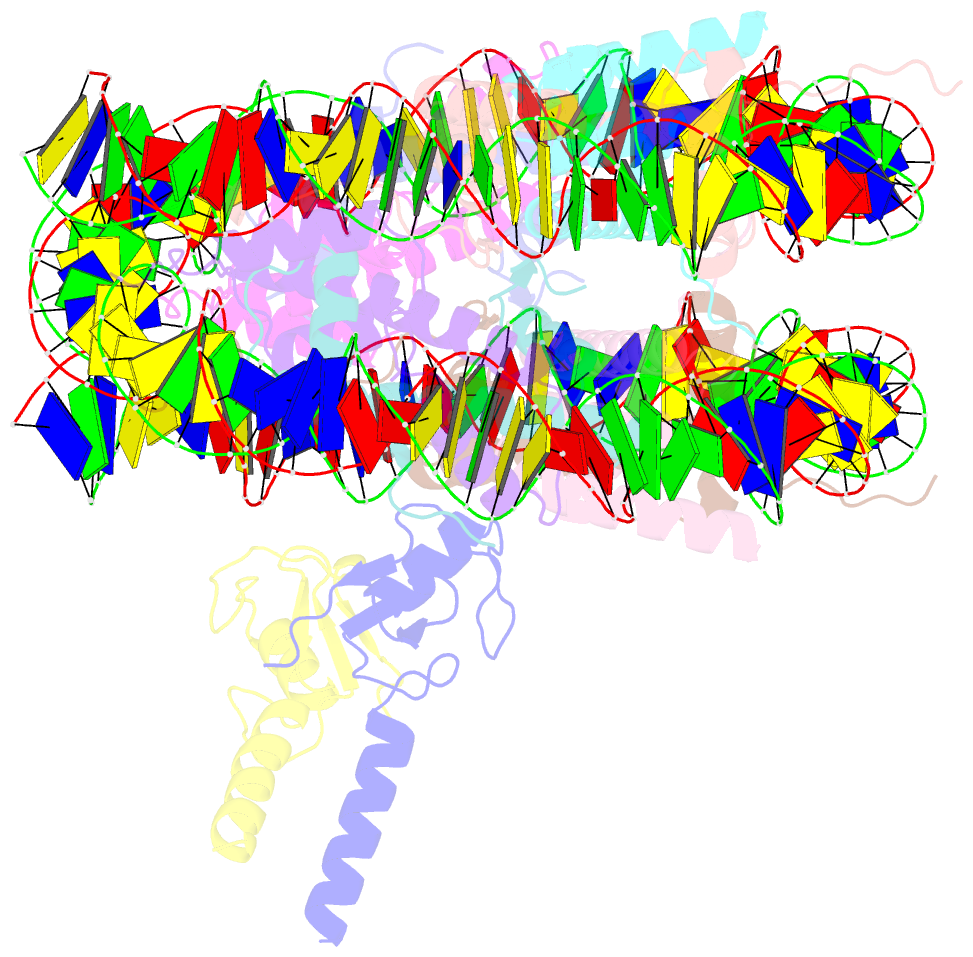
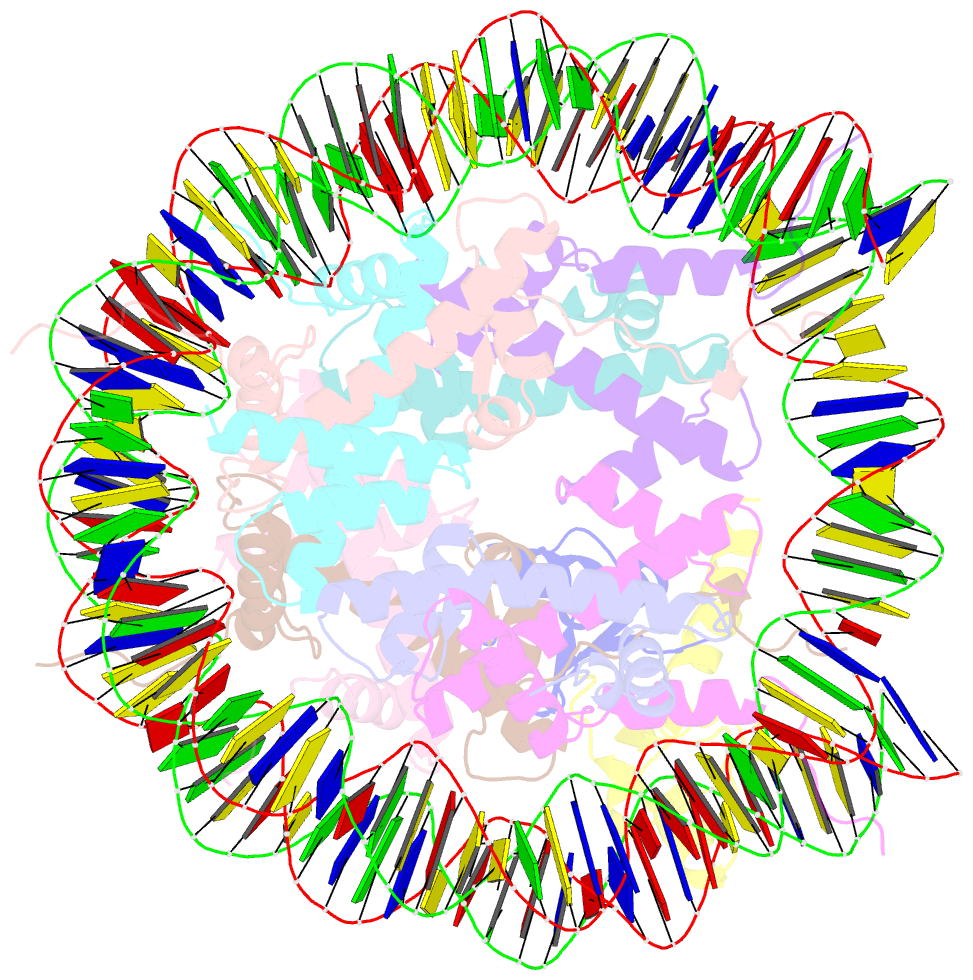
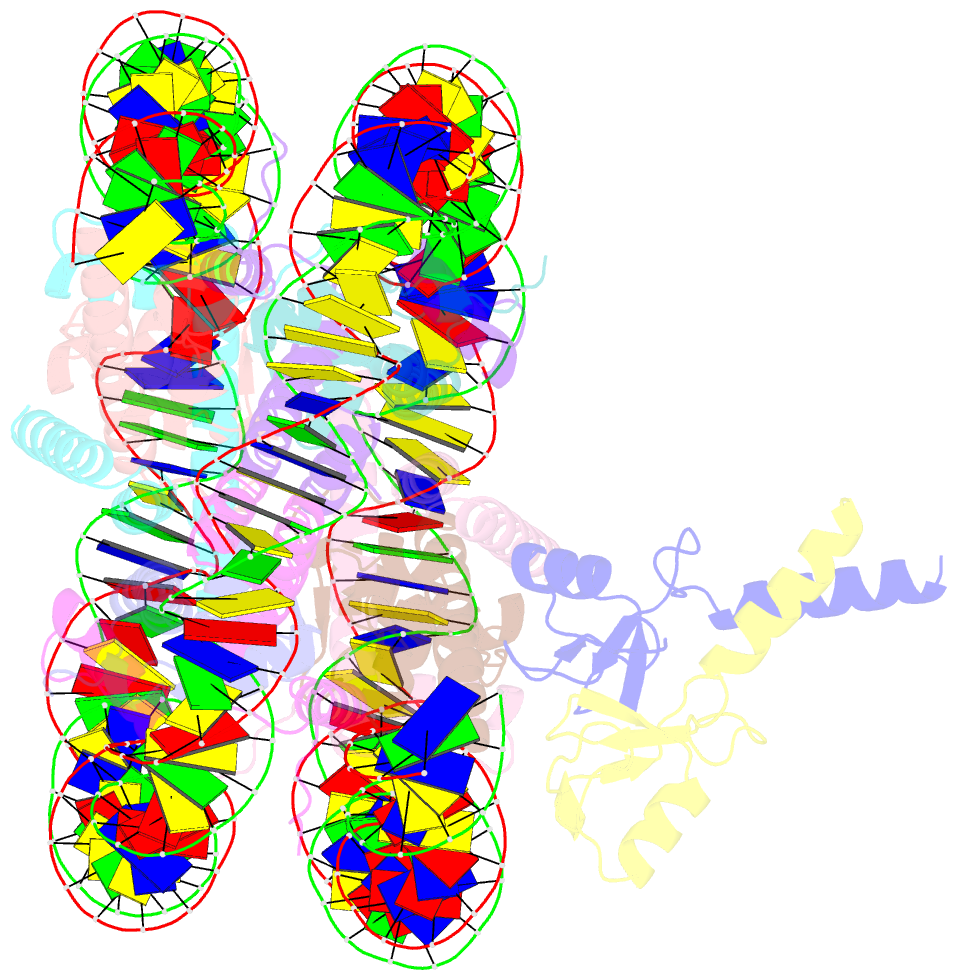
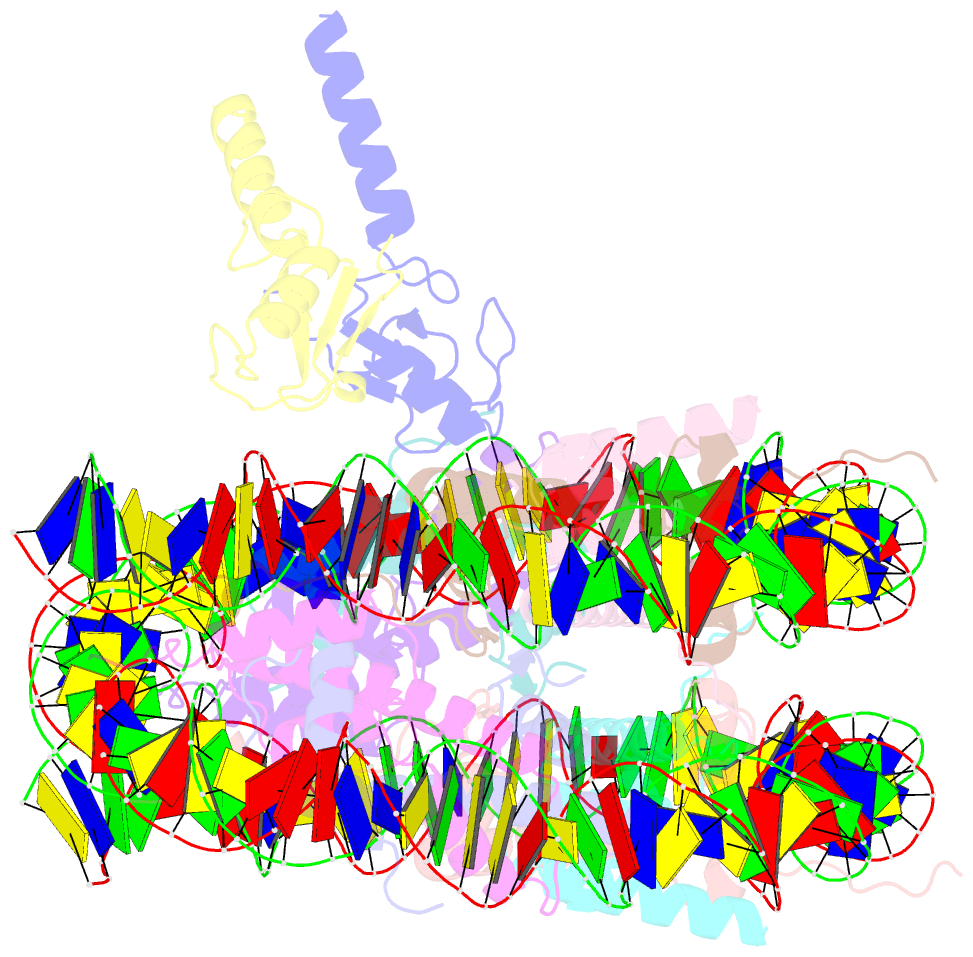
- Bre1-nucleosome complex (model ii)
- Reference
- Onishi S, Uchiyama K, Sato K, Okada C, Kobayashi S, Hamada K, Nishizawa T, Nureki O, Ogata K, Sengoku T (2024): "Structure of the human Bre1 complex bound to the nucleosome." Nat Commun, 15, 2580. doi: 10.1038/s41467-024-46910-8.
- Abstract
- Histone H2B monoubiquitination (at Lys120 in humans) regulates transcription elongation and DNA repair. In humans, H2B monoubiquitination is catalyzed by the heterodimeric Bre1 complex composed of Bre1A/RNF20 and Bre1B/RNF40. The Bre1 proteins generally function as tumor suppressors, while in certain cancers, they facilitate cancer cell proliferation. To obtain structural insights of H2BK120 ubiquitination and its regulation, we report the cryo-electron microscopy structure of the human Bre1 complex bound to the nucleosome. The two RING domains of Bre1A and Bre1B recognize the acidic patch and the nucleosomal DNA phosphates around SHL 6.0-6.5, which are ideally located to recruit the E2 enzyme and ubiquitin for H2BK120-specific ubiquitination. Mutational experiments suggest that the two RING domains bind in two orientations and that ubiquitination occurs when Bre1A binds to the acidic patch. Our results provide insights into the H2BK120-specific ubiquitination by the Bre1 proteins and suggest that H2B monoubiquitination can be regulated by nuclesomal DNA flexibility.