Summary information and primary citation
- PDB-id
- 8gzp; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- viral protein
- Method
- cryo-EM (3.6 Å)
- Summary
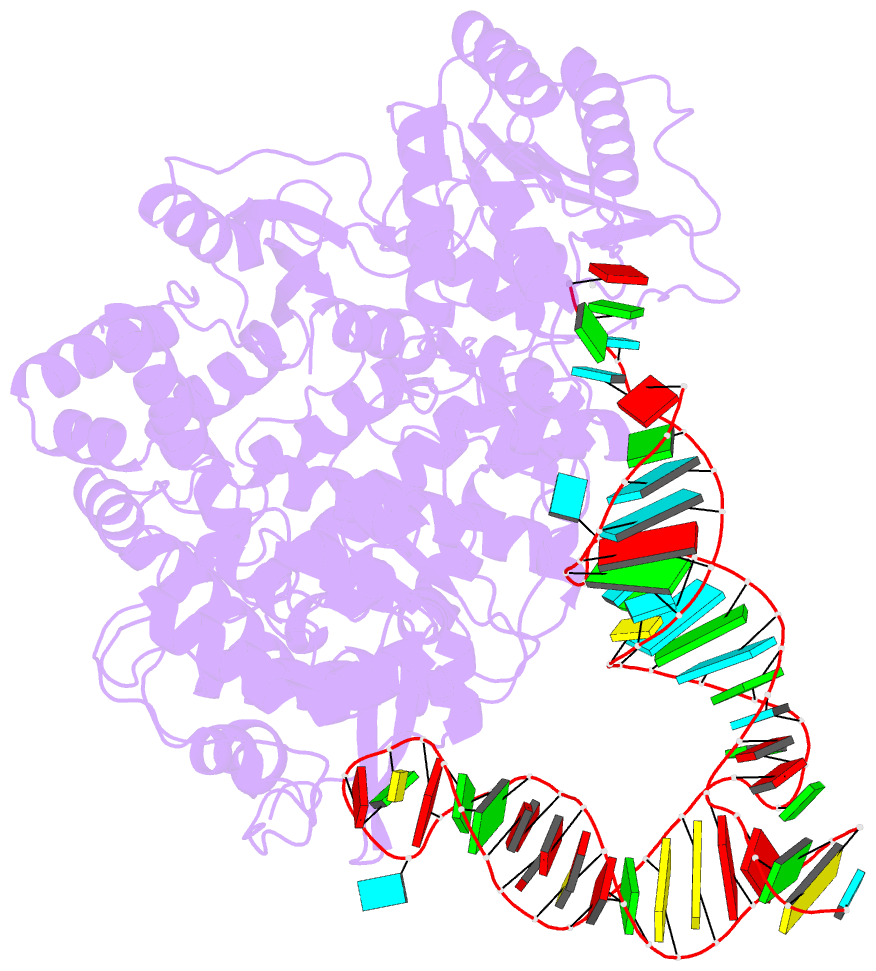
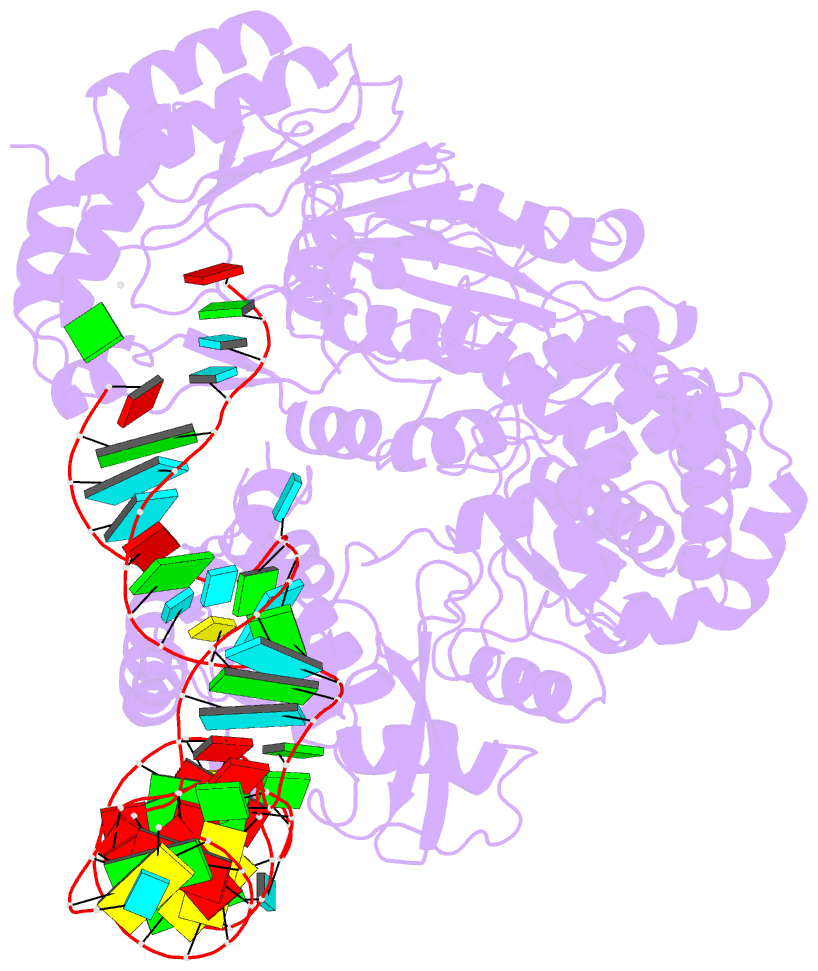
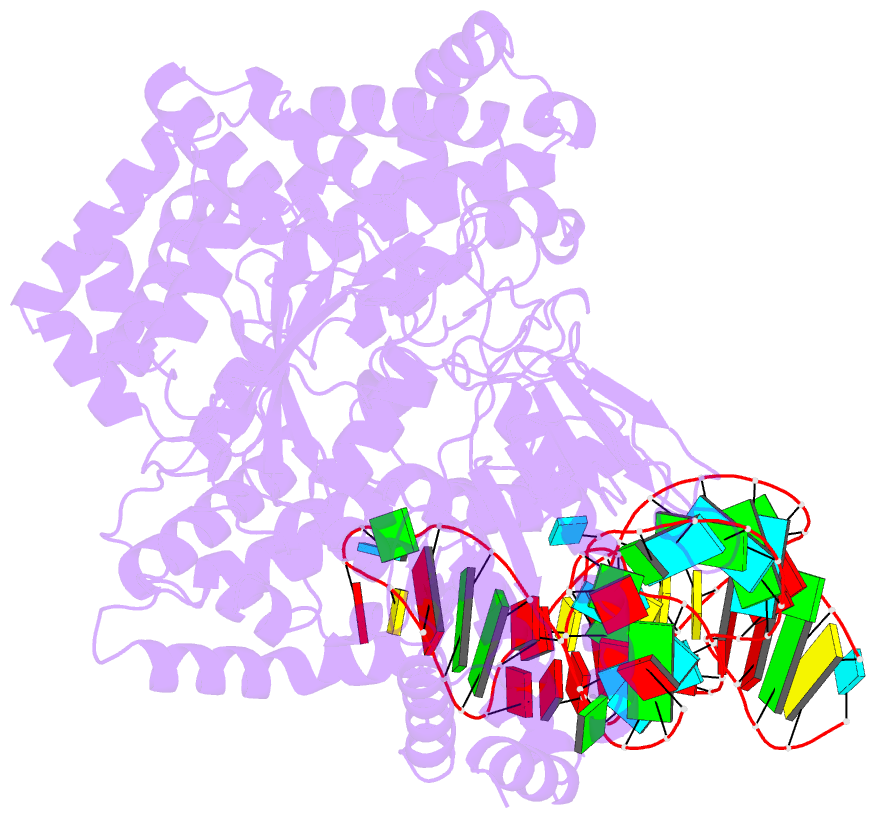
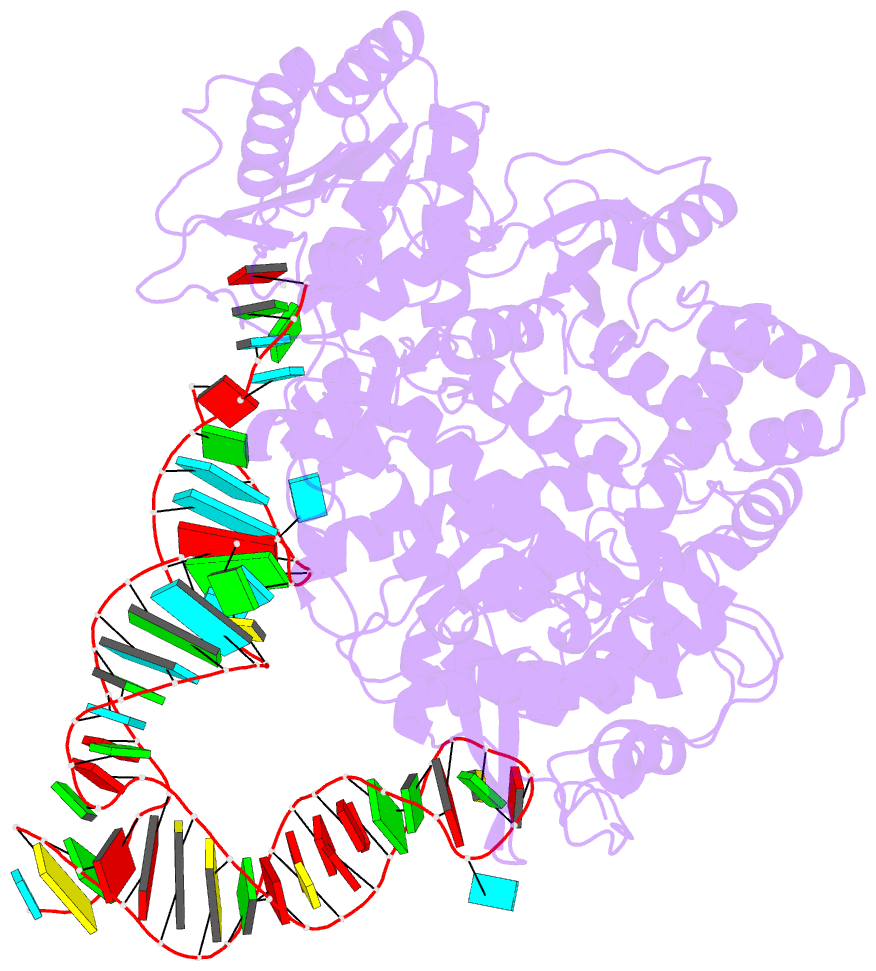
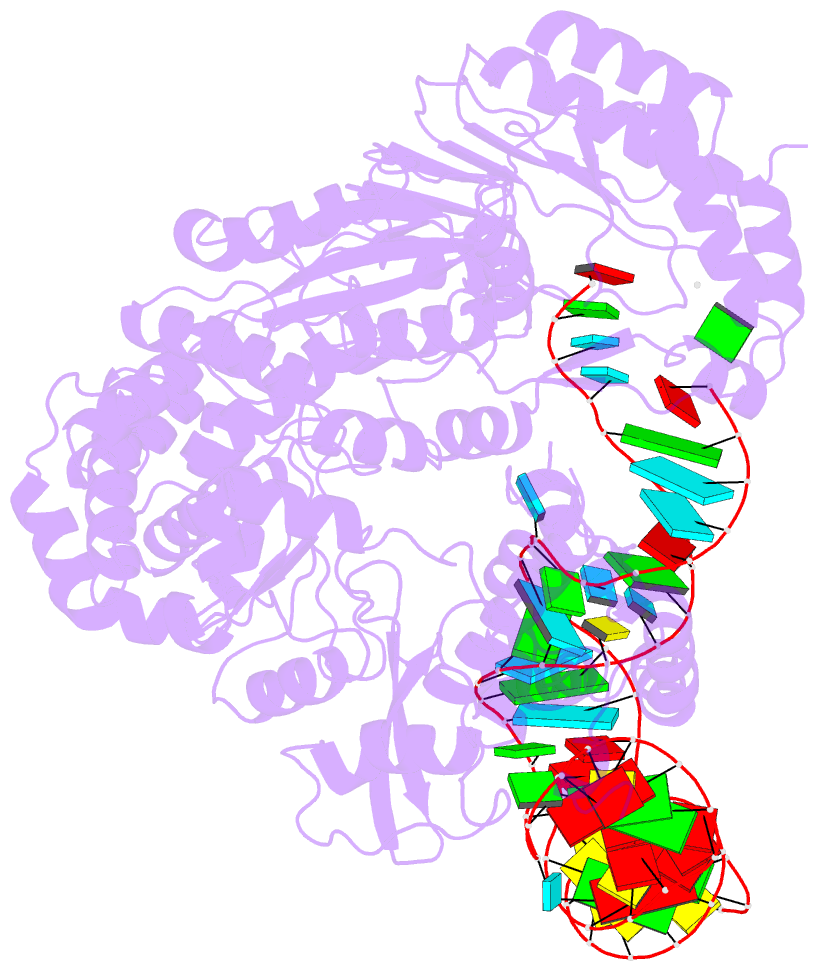
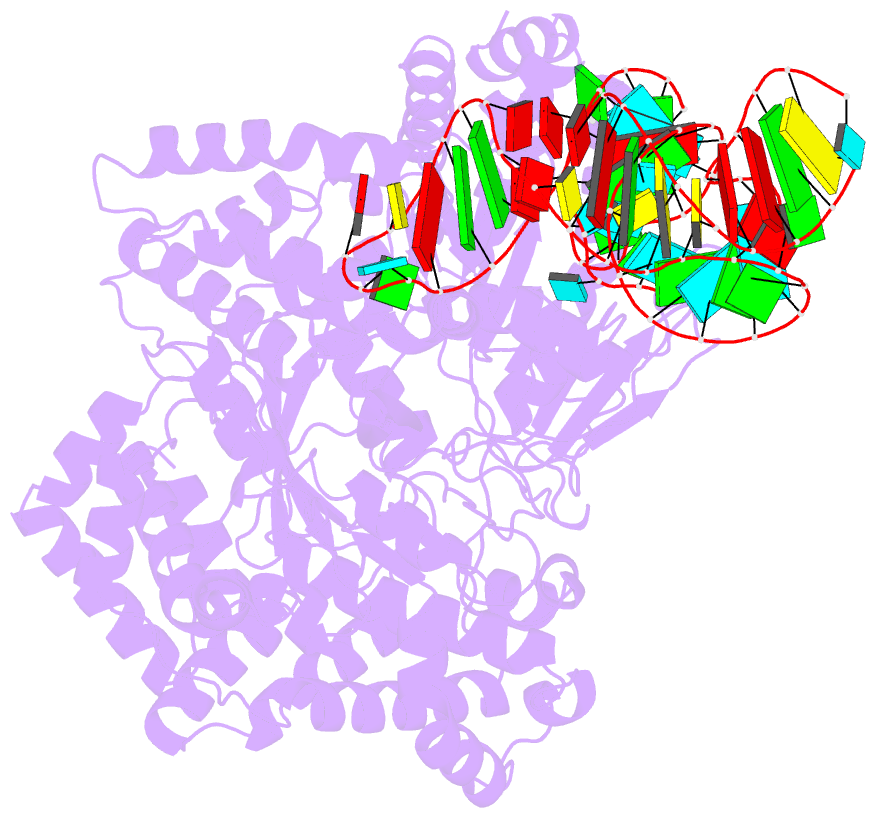
- cryo-EM structure of the ns5-sla complex
- Reference
- Osawa T, Aoki M, Ehara H, Sekine SI (2023): "Structures of dengue virus RNA replicase complexes." Mol.Cell, 83, 2781. doi: 10.1016/j.molcel.2023.06.023.
- Abstract
- Dengue is a mosquito-borne viral infection caused by dengue virus (DENV), a member of the flaviviruses. The DENV genome is a 5'-capped positive-sense RNA with a unique 5'-stem-loop structure (SLA), which is essential for RNA replication and 5' capping. The virus-encoded proteins NS5 and NS3 are responsible for viral genome replication, but the structural basis by which they cooperatively conduct the required tasks has remained unclear. Here, we report the cryoelectron microscopy (cryo-EM) structures of SLA-bound NS5 (PC), NS3-bound PC (PC-NS3), and an RNA-elongating NS5-NS3 complex (EC). While SLA bridges the NS5 methyltransferase and RNA-dependent RNA polymerase domains in PC, the NS3 helicase domain displaces it in elongation complex (EC). The SLA- and NS3-binding sites overlap with that of human STAT2. These structures illuminate the key steps in DENV genome replication, namely, SLA-dependent replication initiation, processive RNA elongation, and 5' capping of the nascent genomic RNA, thereby providing foundations to combat flaviviruses.