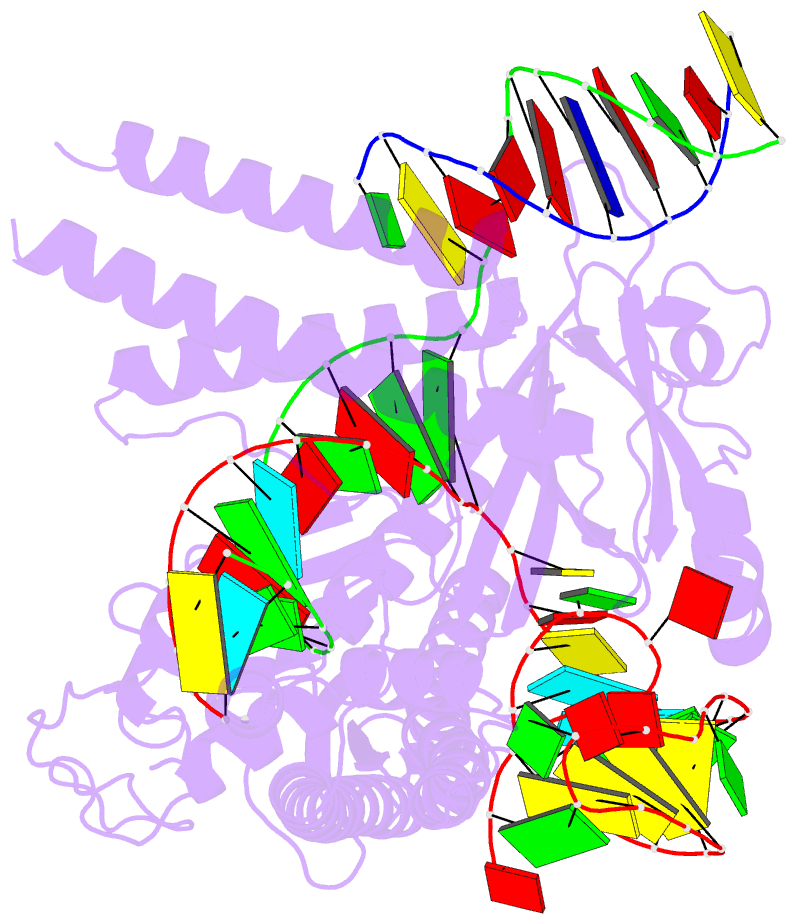
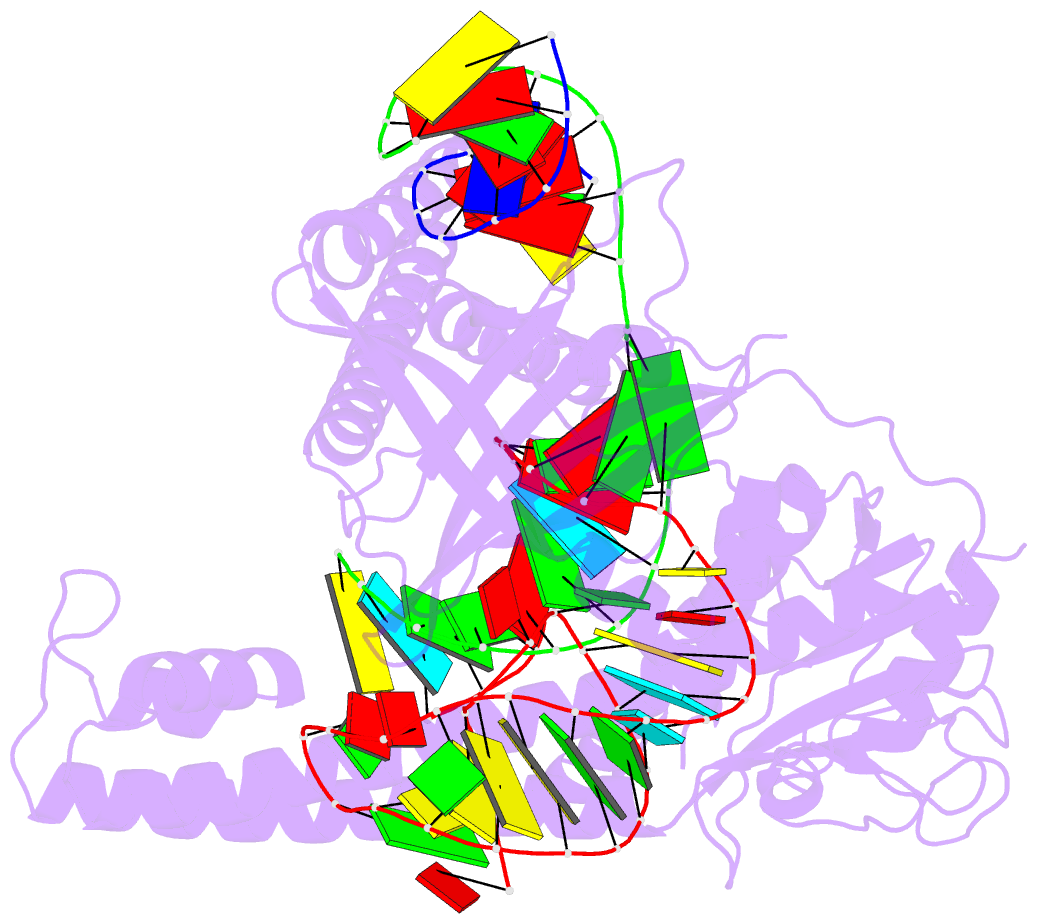
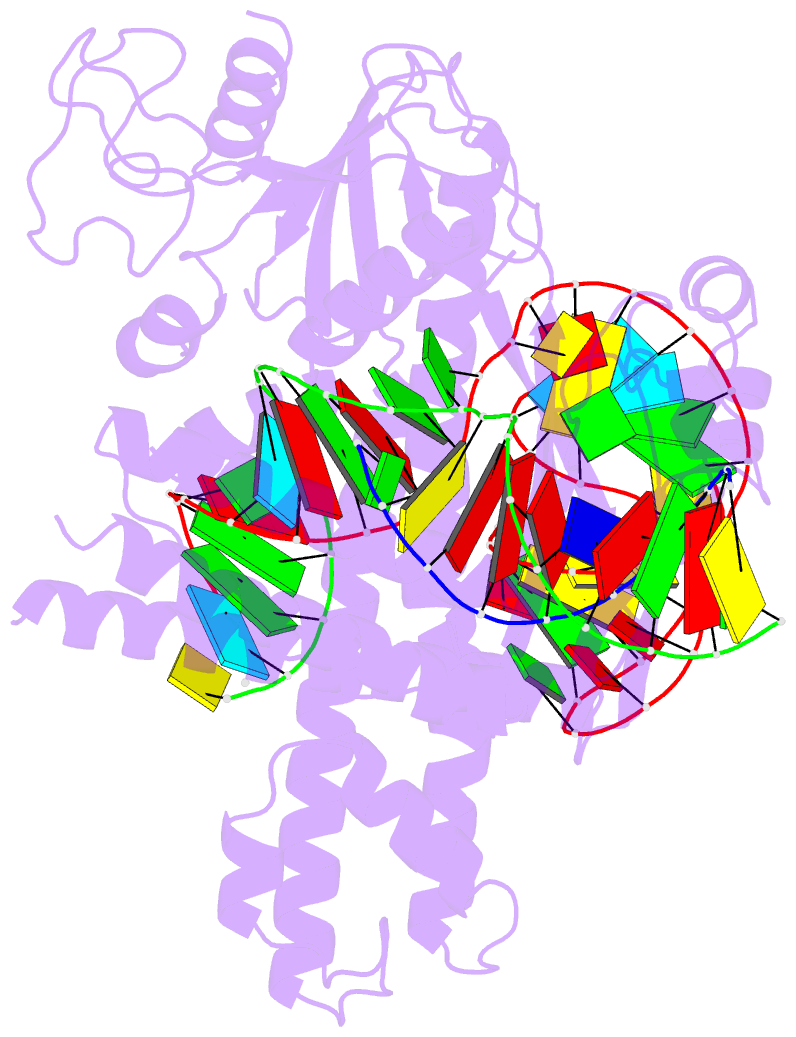
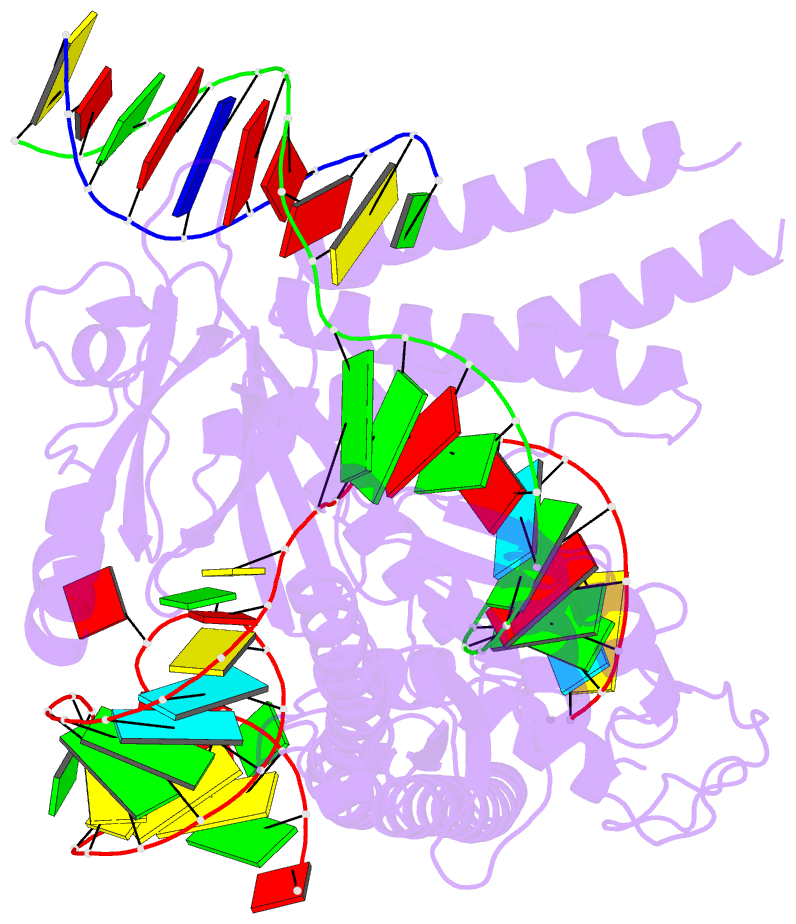
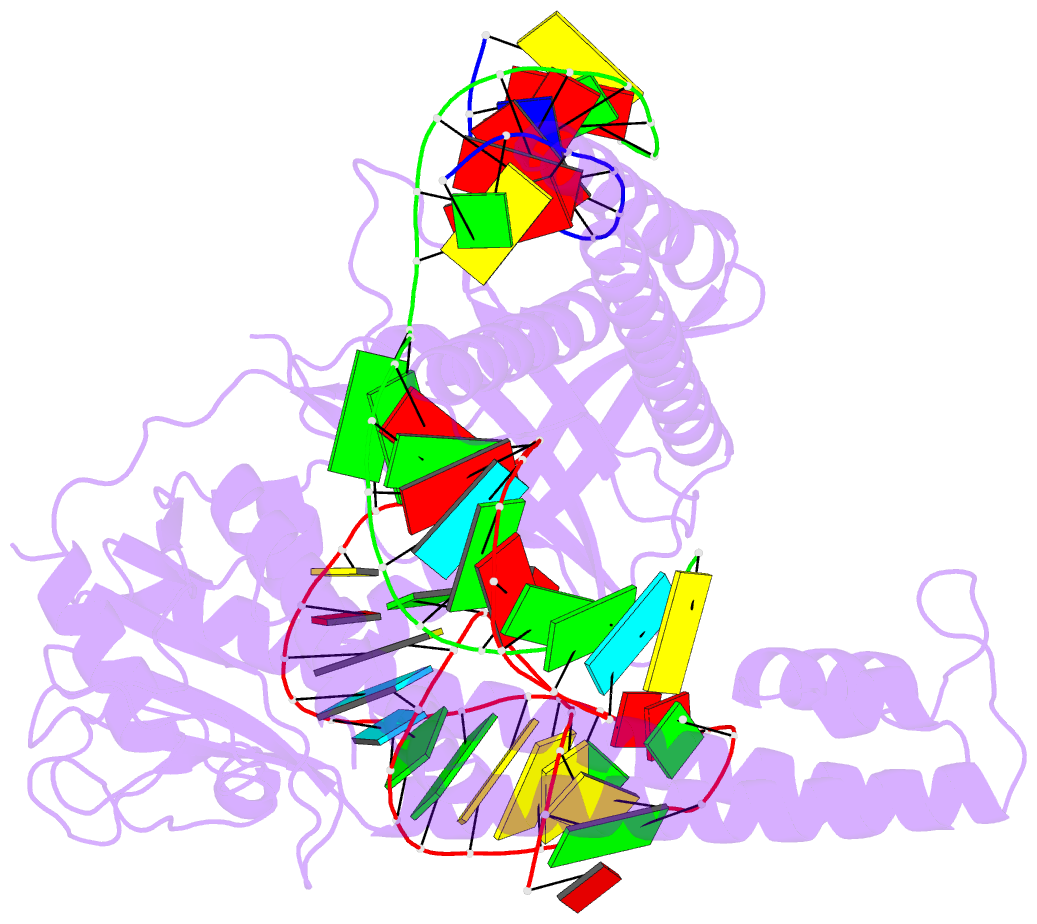
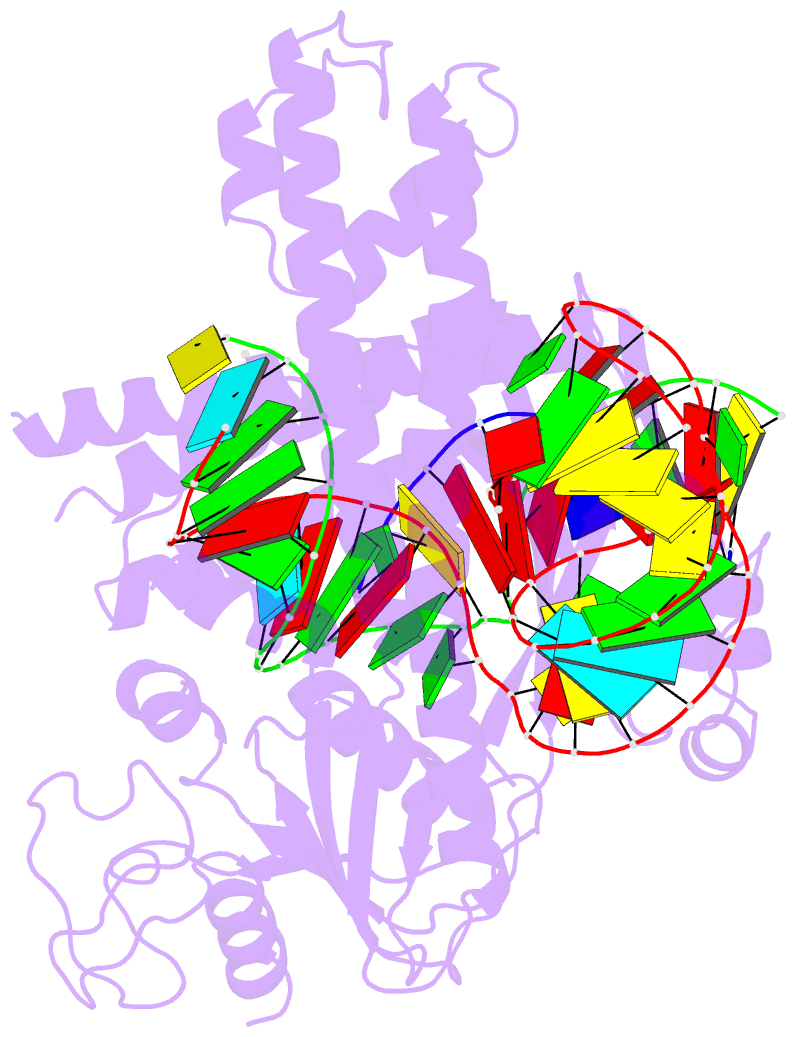
Summary information and primary citation
- PDB-id
- 8hhm; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein
- Method
- cryo-EM (3.08 Å)
- Summary
- cryo-EM structure of the cas12m2-crrna-target DNA ternary complex intermediate state
- Reference
- Omura SN, Nakagawa R, Sudfeld C, Villegas Warren R, Wu WY, Hirano H, Laffeber C, Kusakizako T, Kise Y, Lebbink JHG, Itoh Y, van der Oost J, Nureki O (2023): "Mechanistic and evolutionary insights into a type V-M CRISPR-Cas effector enzyme." Nat.Struct.Mol.Biol., 30, 1172-1182. doi: 10.1038/s41594-023-01042-3.
- Abstract
- RNA-guided type V CRISPR-Cas12 effectors provide adaptive immunity against mobile genetic elements (MGEs) in bacteria and archaea. Among diverse Cas12 enzymes, the recently identified Cas12m2 (CRISPR-Cas type V-M) is highly compact and has a unique RuvC active site. Although the non-canonical RuvC triad does not permit dsDNA cleavage, Cas12m2 still protects against invading MGEs through transcriptional silencing by strong DNA binding. However, the molecular mechanism of RNA-guided genome inactivation by Cas12m2 remains unknown. Here we report cryo-electron microscopy structures of two states of Cas12m2-CRISPR RNA (crRNA)-target DNA ternary complexes and the Cas12m2-crRNA binary complex, revealing structural dynamics during crRNA-target DNA heteroduplex formation. The structures indicate that the non-target DNA strand is tightly bound to a unique arginine-rich cluster in the recognition (REC) domains and the non-canonical active site in the RuvC domain, ensuring strong DNA-binding affinity of Cas12m2. Furthermore, a structural comparison of Cas12m2 with TnpB, a putative ancestor of Cas12 enzymes, suggests that the interaction of the characteristic coiled-coil REC2 insertion with the protospacer-adjacent motif-distal region of the heteroduplex is crucial for Cas12m2 to engage in adaptive immunity. Collectively, our findings improve mechanistic understanding of diverse type V CRISPR-Cas effectors and provide insights into the evolution of TnpB to Cas12 enzymes.