Summary information and primary citation
- PDB-id
- 8hmy; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- cryo-EM (2.94 Å)
- Summary
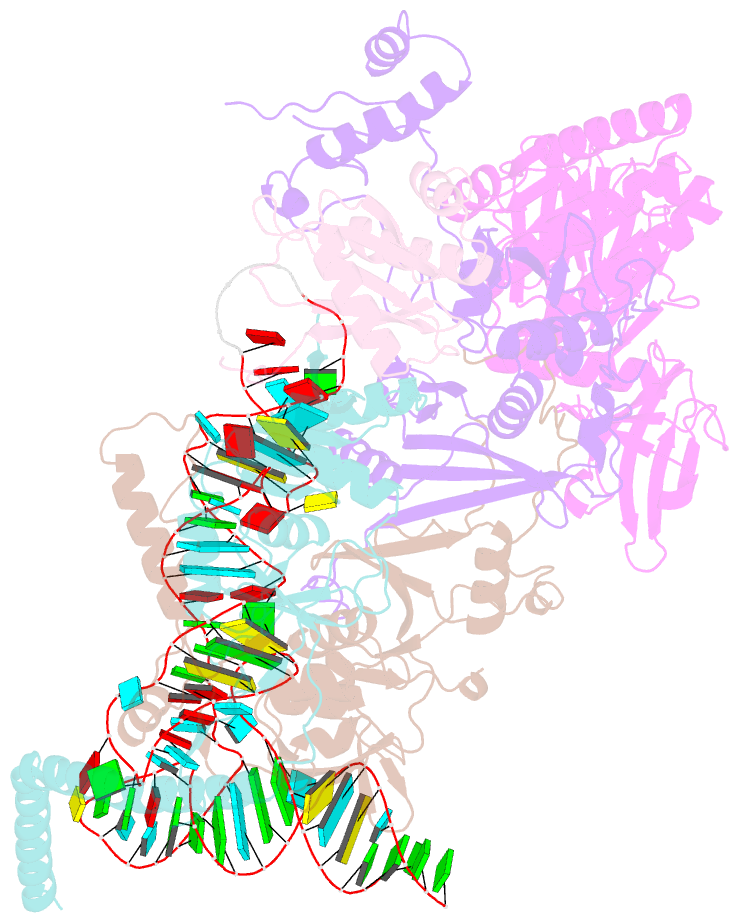
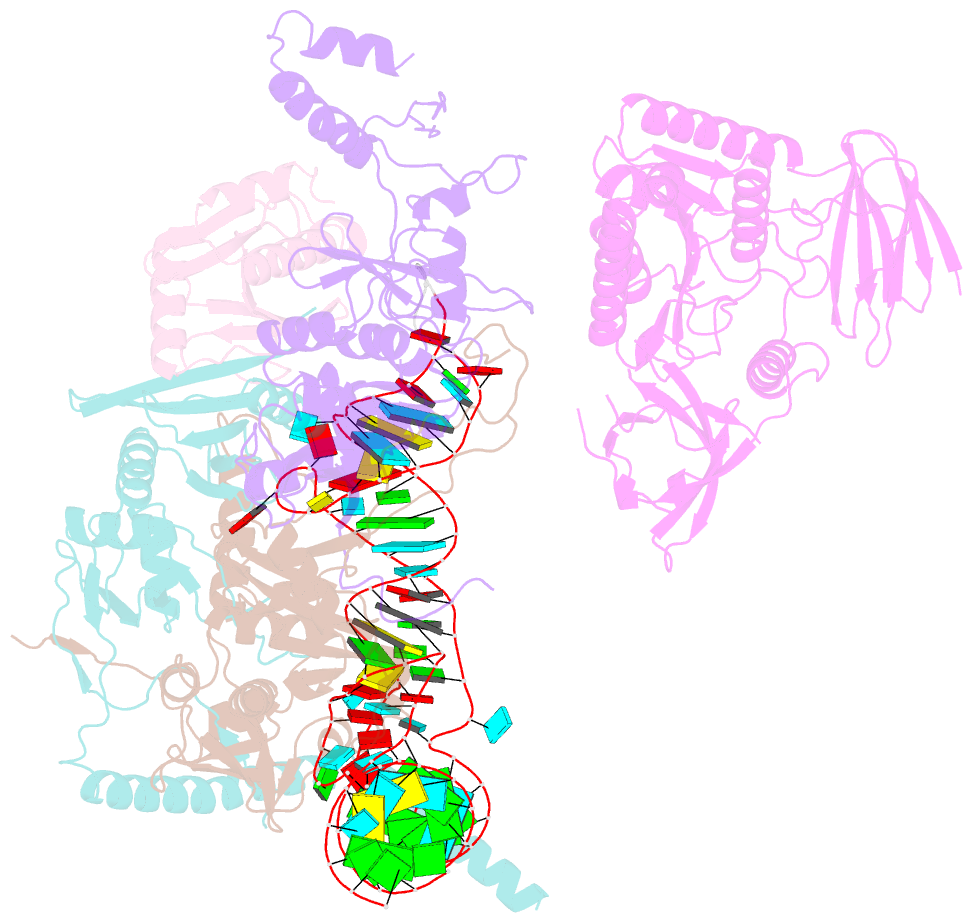
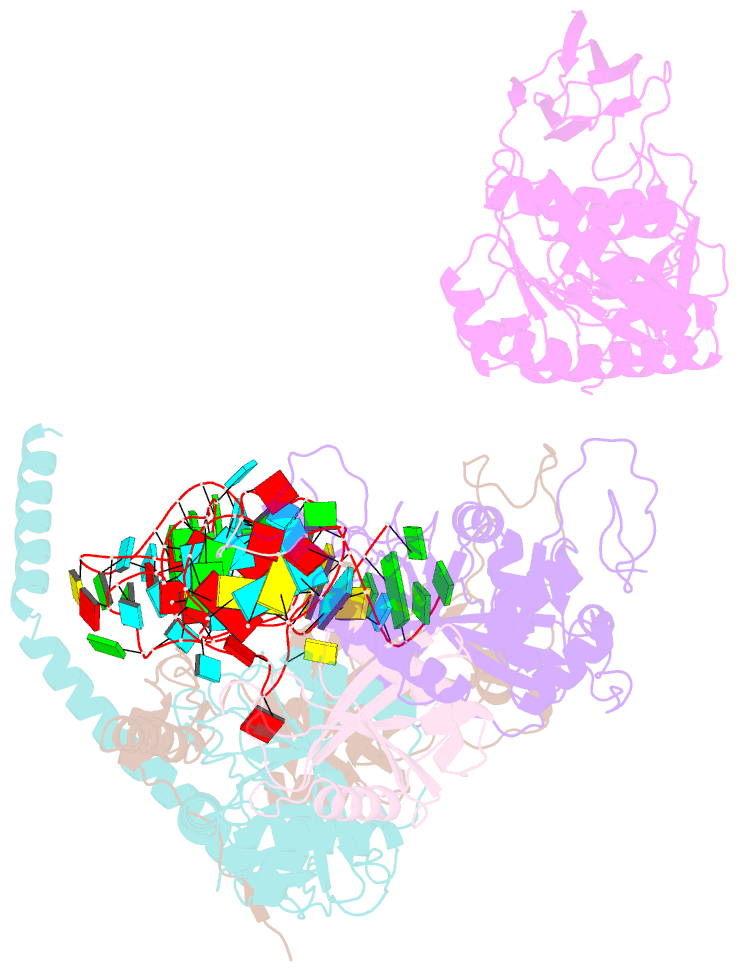
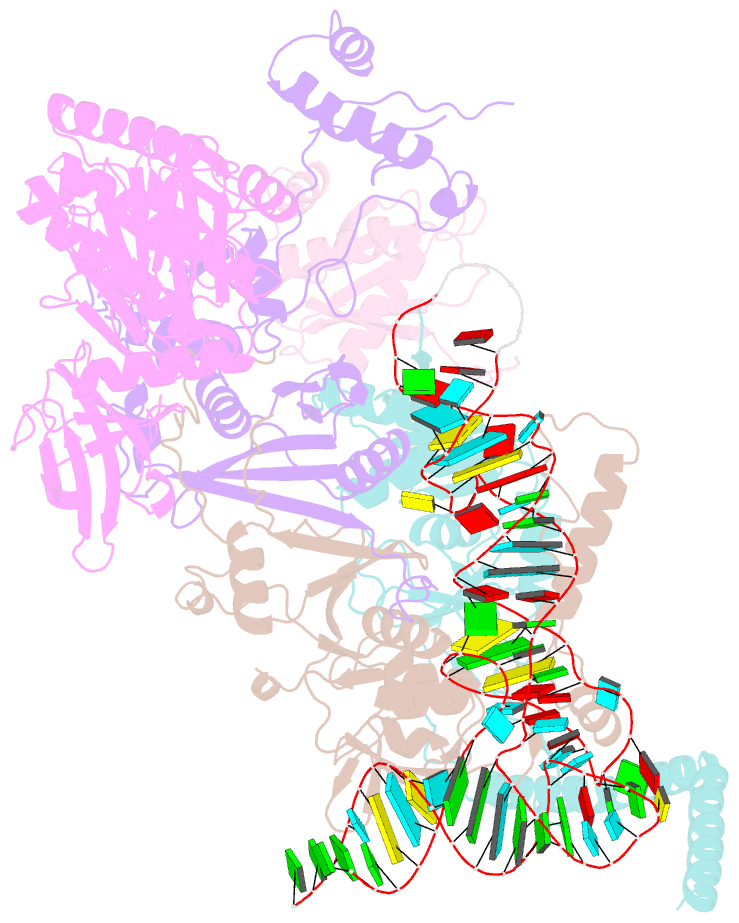
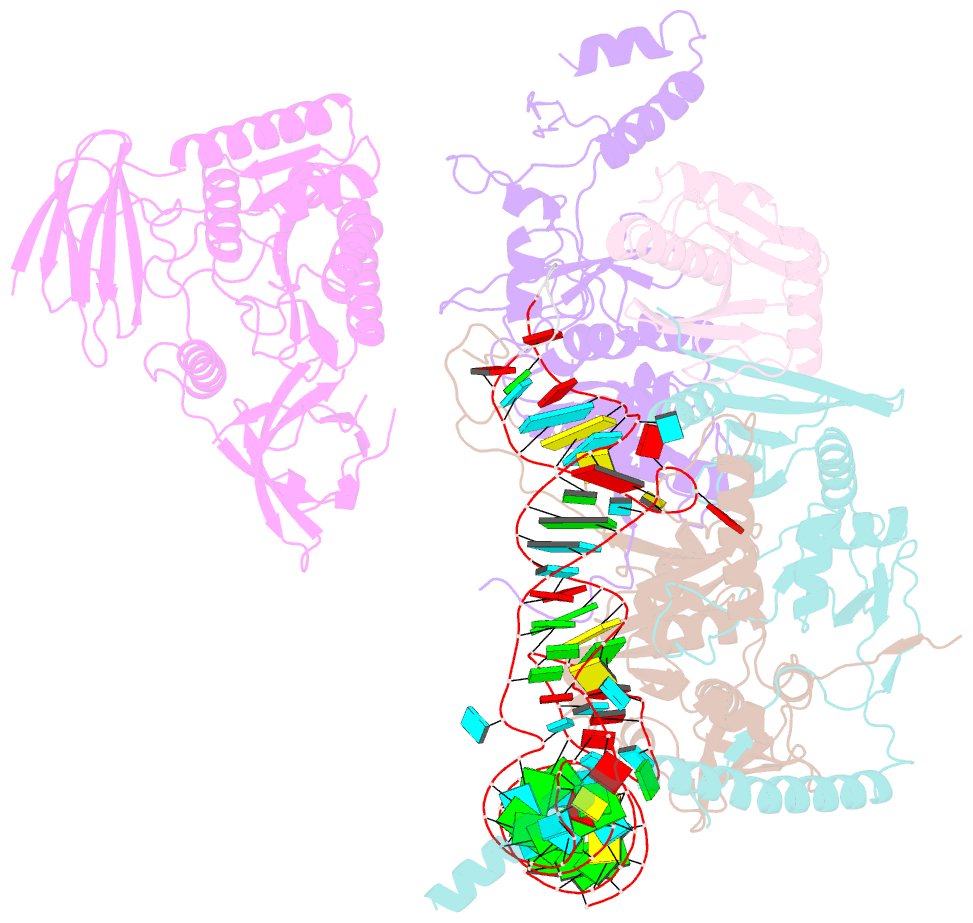
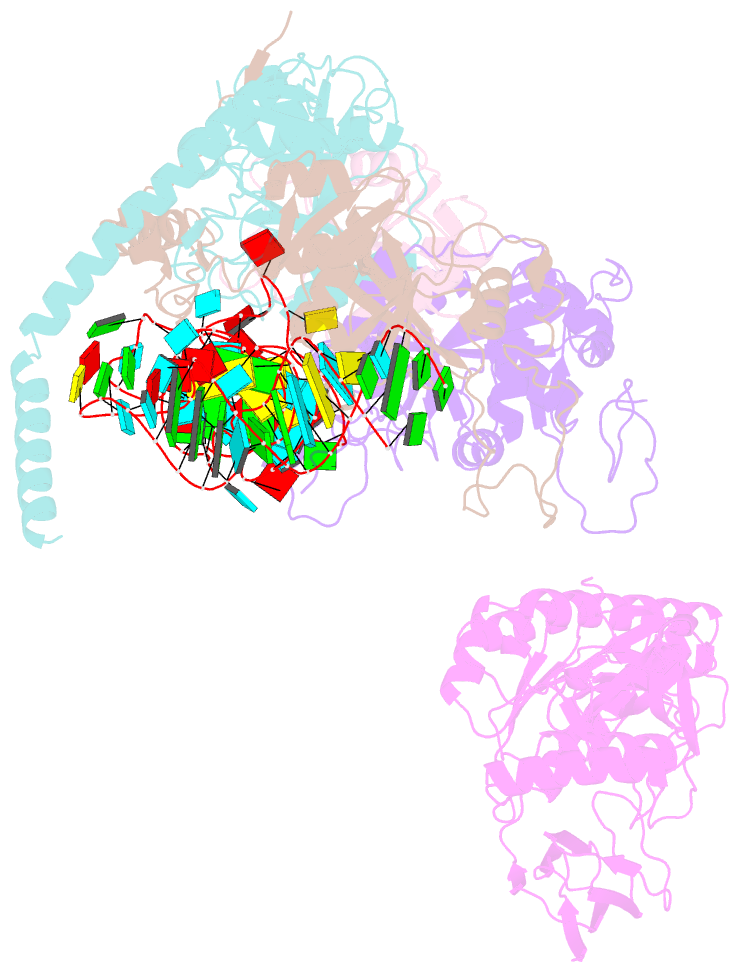
- cryo-EM structure of the human pre-catalytic tsen-pre-trna complex
- Reference
- Zhang X, Yang F, Zhan X, Bian T, Xing Z, Lu Y, Shi Y (2023): "Structural basis of pre-tRNA intron removal by human tRNA splicing endonuclease." Mol.Cell, 83, 1328-1339.e4. doi: 10.1016/j.molcel.2023.03.015.
- Abstract
- Removal of the intron from precursor-tRNA (pre-tRNA) is essential in all three kingdoms of life. In humans, this process is mediated by the tRNA splicing endonuclease (TSEN) comprising four subunits: TSEN2, TSEN15, TSEN34, and TSEN54. Here, we report the cryo-EM structures of human TSEN bound to full-length pre-tRNA in the pre-catalytic and post-catalytic states at average resolutions of 2.94 and 2.88 Å, respectively. Human TSEN features an extended surface groove that holds the L-shaped pre-tRNA. The mature domain of pre-tRNA is recognized by conserved structural elements of TSEN34, TSEN54, and TSEN2. Such recognition orients the anticodon stem of pre-tRNA and places the 3'-splice site and 5'-splice site into the catalytic centers of TSEN34 and TSEN2, respectively. The bulk of the intron sequences makes no direct interaction with TSEN, explaining why pre-tRNAs of varying introns can be accommodated and cleaved. Our structures reveal the molecular ruler mechanism of pre-tRNA cleavage by TSEN.