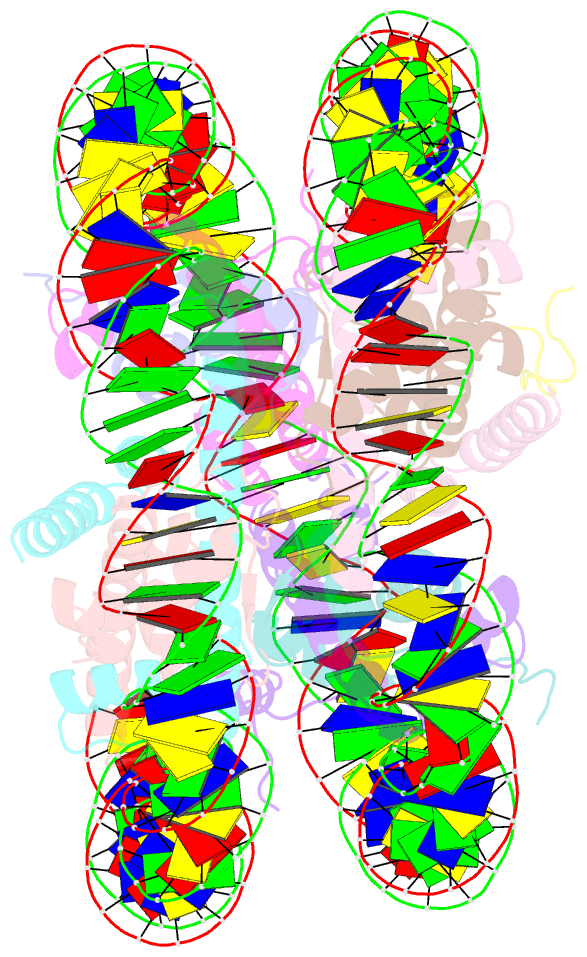
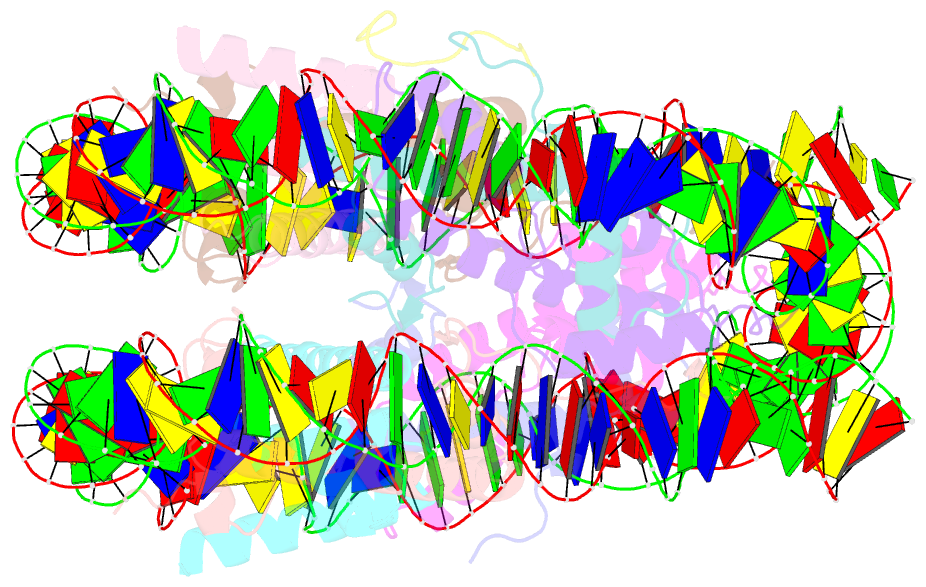
Summary information and primary citation
- PDB-id
- 8hr1; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- structural protein
- Method
- cryo-EM (3.02 Å)
- Summary
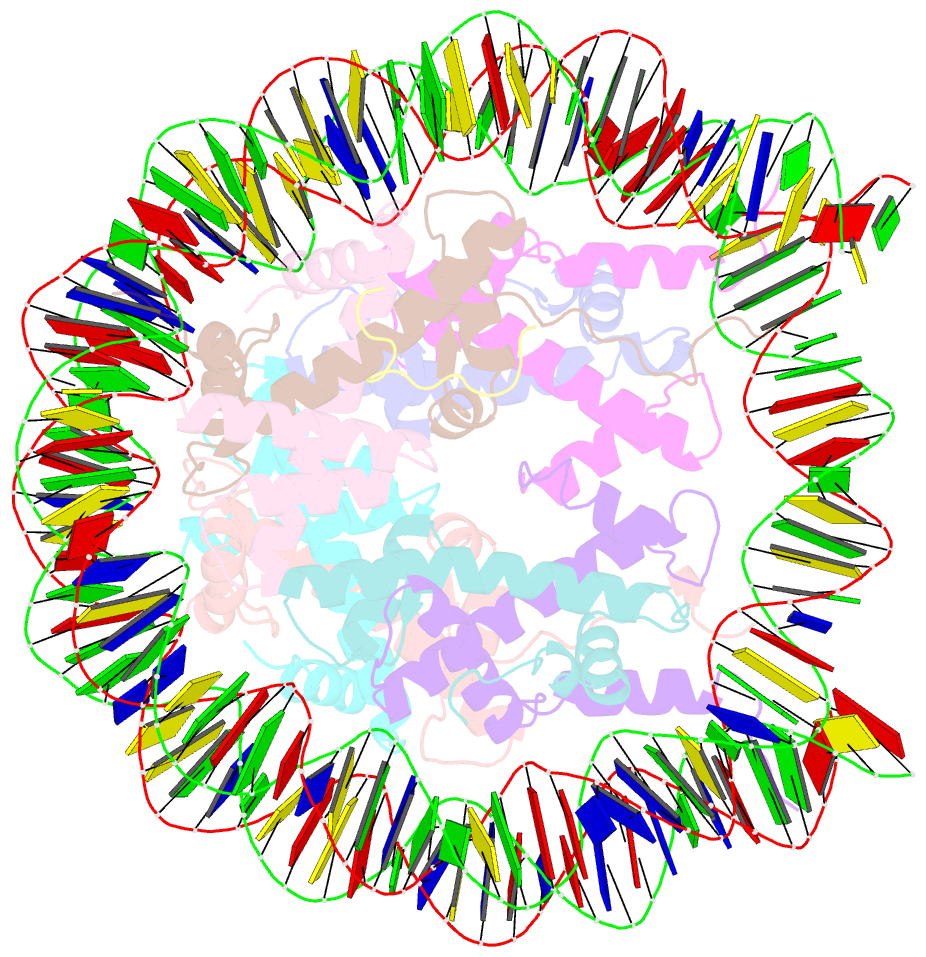
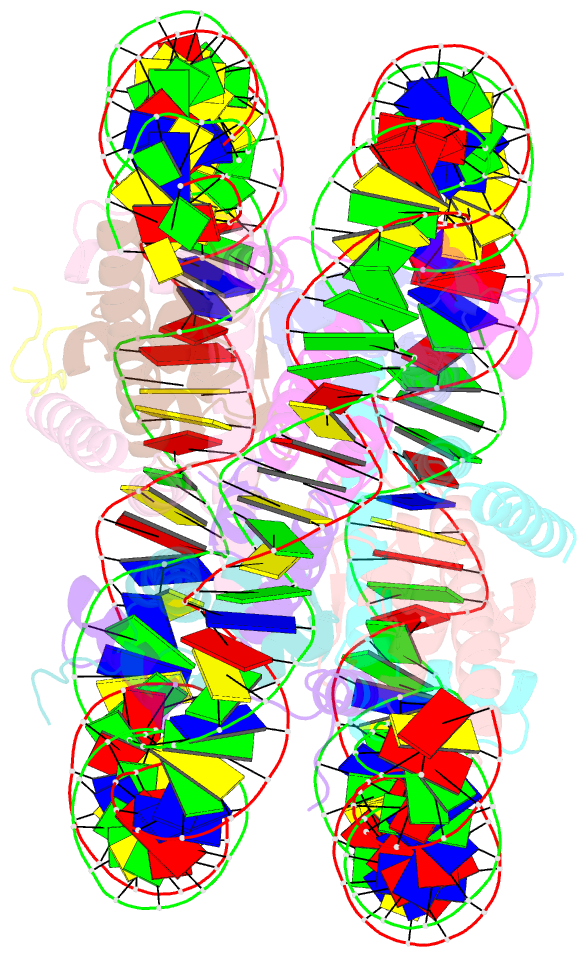
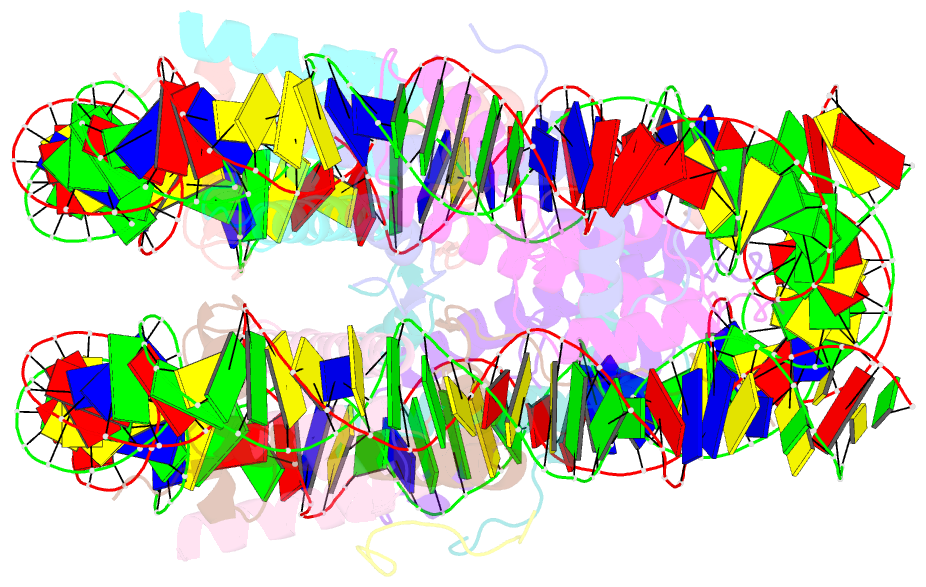
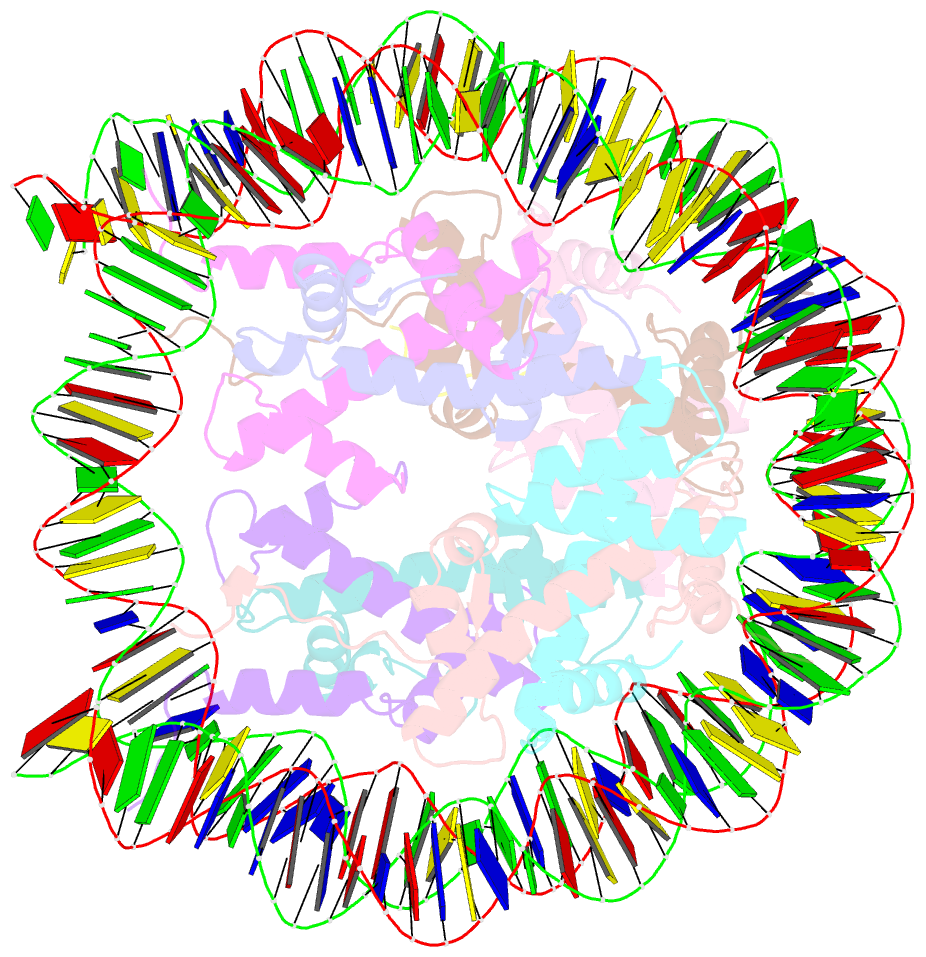
- cryo-EM structure of ssx1 bound to the unmodified nucleosome at a resolution of 3.02 angstrom
- Reference
- Tong Z, Ai H, Xu Z, He K, Chu GC, Shi Q, Deng Z, Xue Q, Sun M, Du Y, Liang L, Li JB, Pan M, Liu L (2024): "Synovial sarcoma X breakpoint 1 protein uses a cryptic groove to selectively recognize H2AK119Ub nucleosomes." Nat.Struct.Mol.Biol., 31, 300-310. doi: 10.1038/s41594-023-01141-1.
- Abstract
- The cancer-specific fusion oncoprotein SS18-SSX1 disturbs chromatin accessibility by hijacking the BAF complex from the promoters and enhancers to the Polycomb-repressed chromatin regions. This process relies on the selective recognition of H2AK119Ub nucleosomes by synovial sarcoma X breakpoint 1 (SSX1). However, the mechanism underlying the selective recognition of H2AK119Ub nucleosomes by SSX1 in the absence of ubiquitin (Ub)-binding capacity remains unknown. Here we report the cryo-EM structure of SSX1 bound to H2AK119Ub nucleosomes at 3.1-Å resolution. Combined in vitro biochemical and cellular assays revealed that the Ub recognition by SSX1 is unique and depends on a cryptic basic groove formed by H3 and the Ub motif on the H2AK119 site. Moreover, this unorthodox binding mode of SSX1 induces DNA unwrapping at the entry/exit sites. Together, our results describe a unique mode of site-specific ubiquitinated nucleosome recognition that underlies the specific hijacking of the BAF complex to Polycomb regions by SS18-SSX1 in synovial sarcoma.