Summary information and primary citation
- PDB-id
- 8hvr; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- gene regulation
- Method
- cryo-EM (3.35 Å)
- Summary
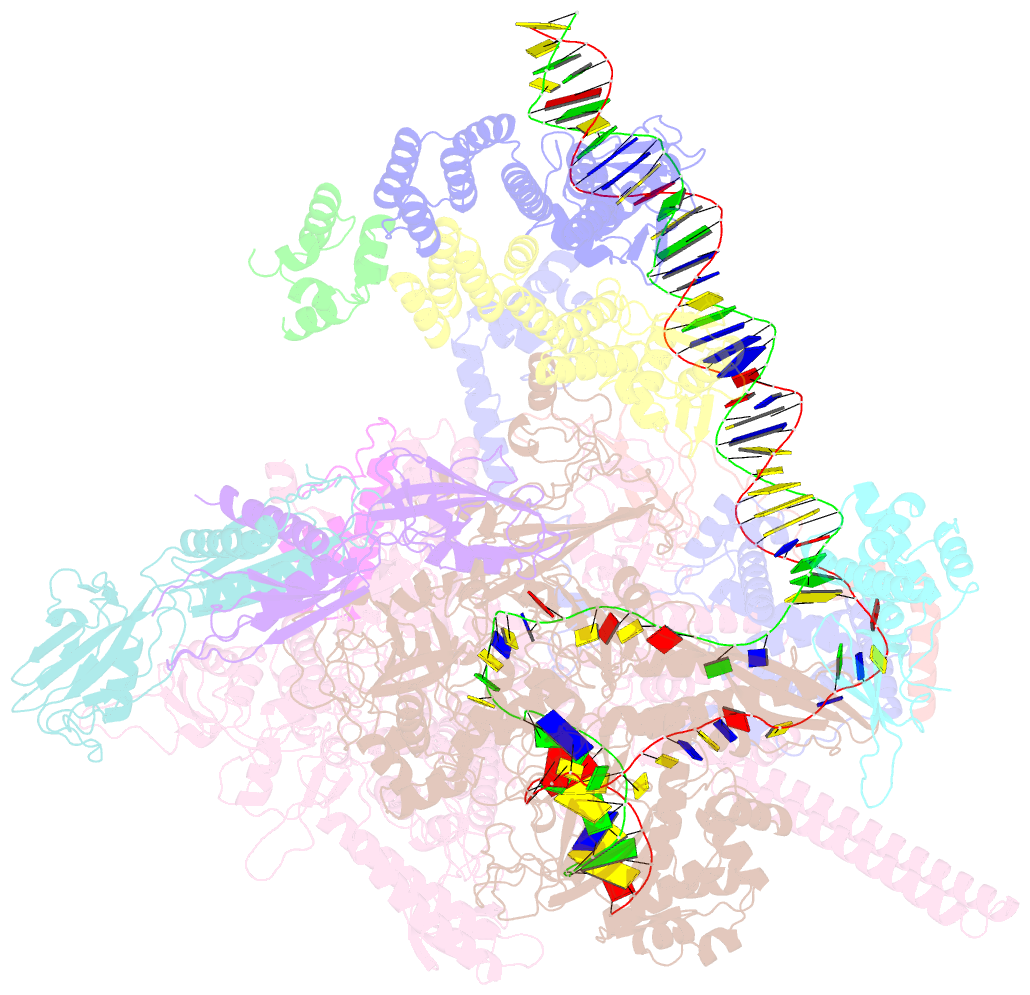
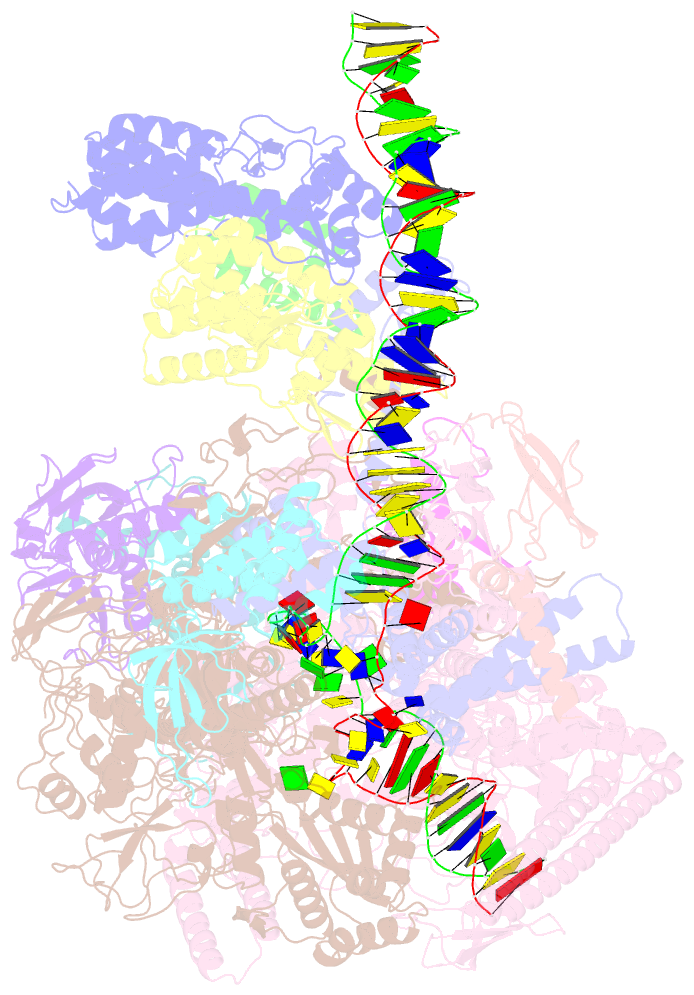
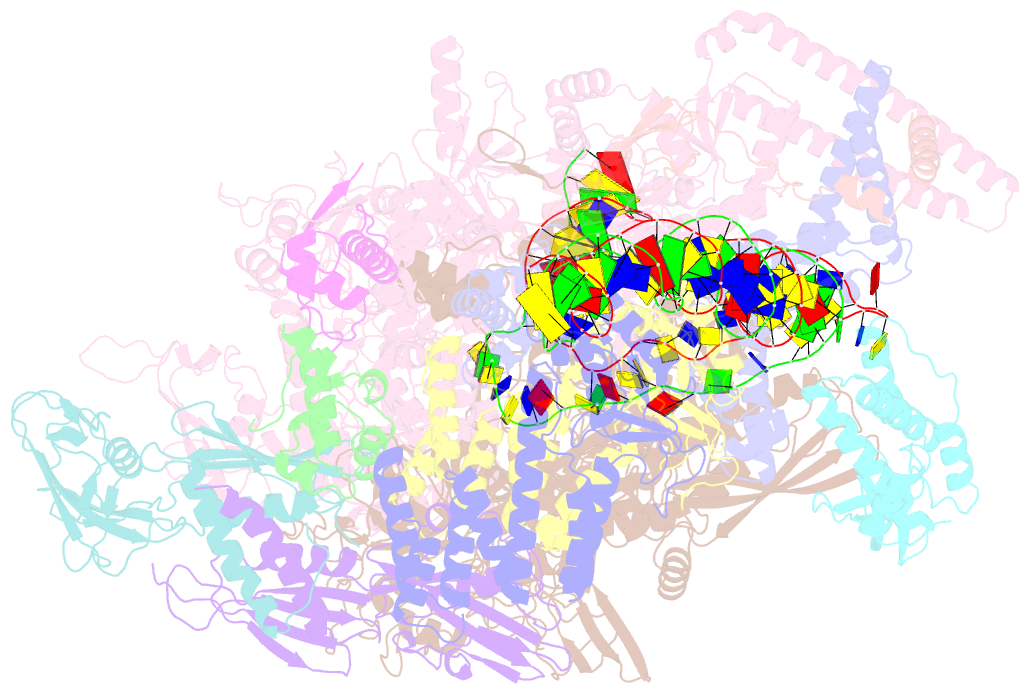
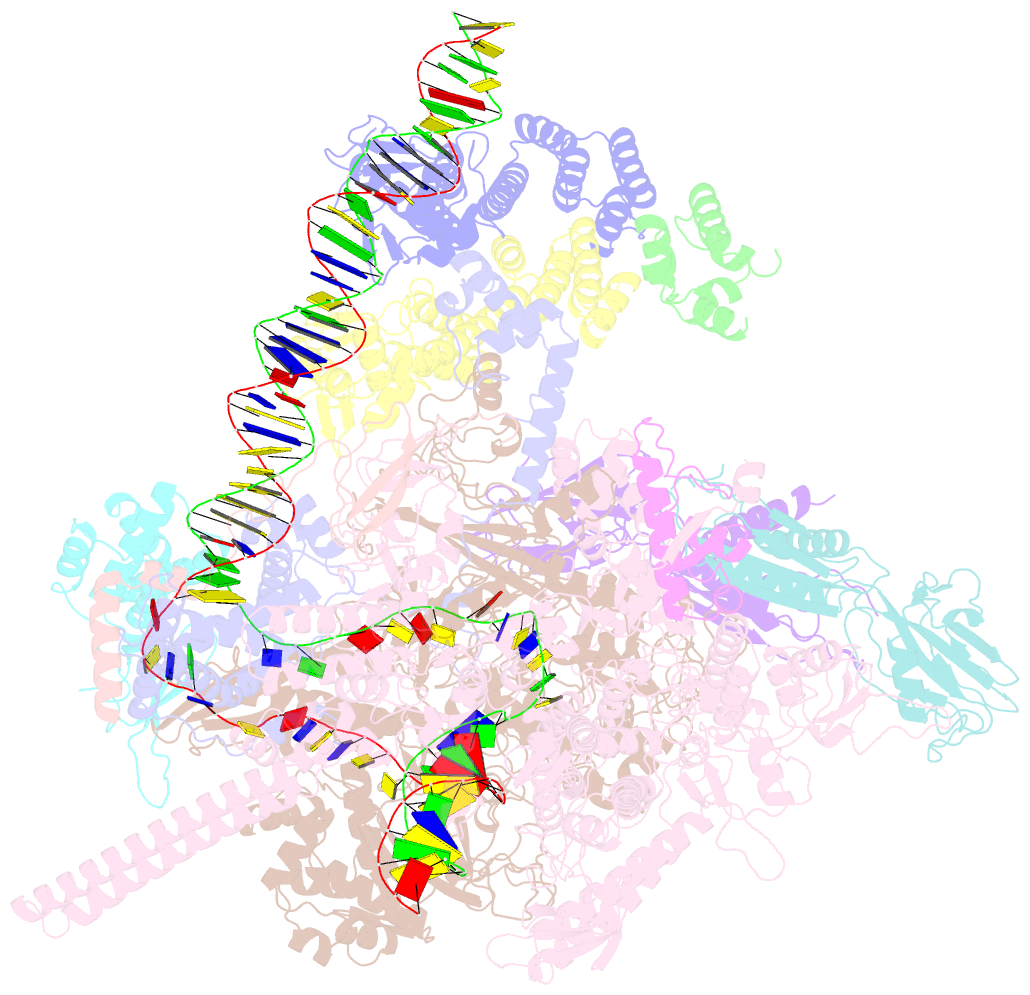
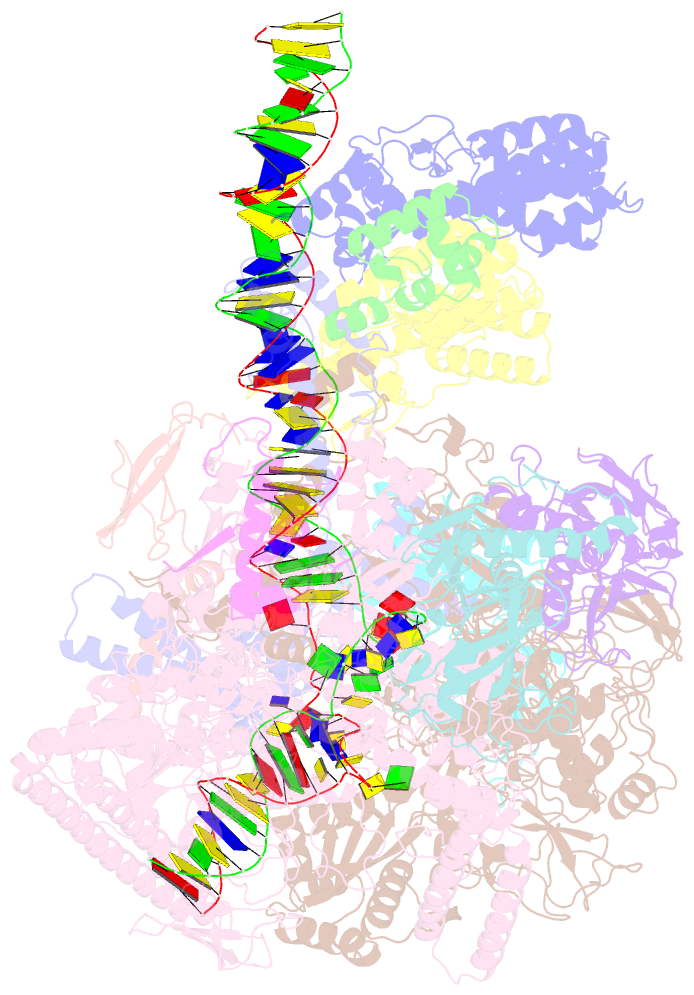
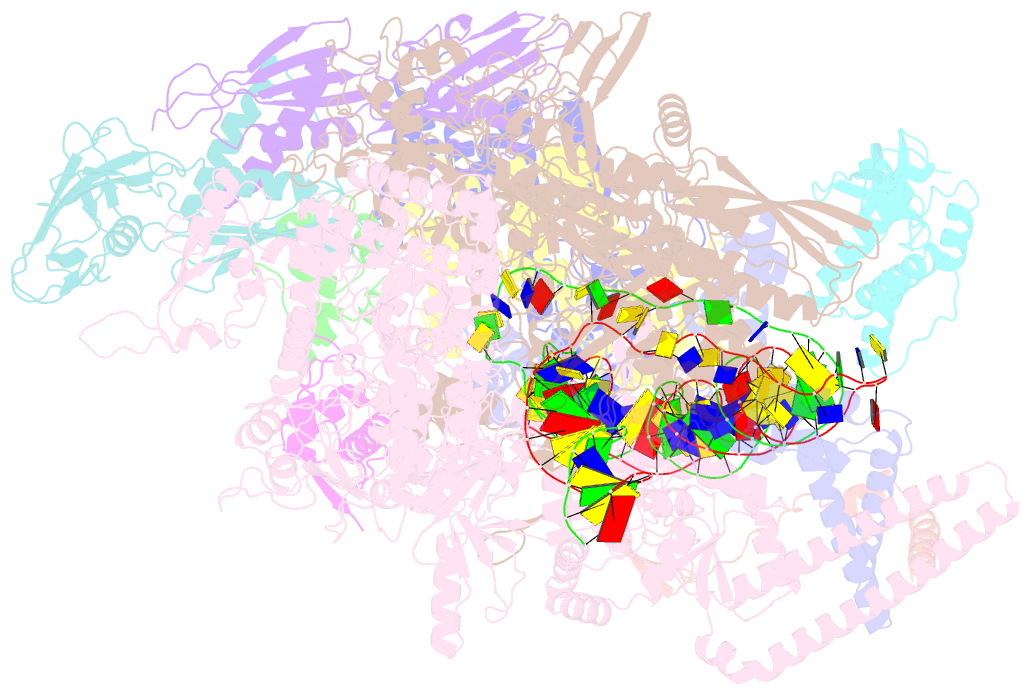
- cryo-EM structure of afsr-dependent transcription activation complex with afss promoter
- Reference
- Wang Y, Yang X, Yu F, Deng Z, Lin S, Zheng J (2024): "Structural and functional characterization of AfsR, an SARP family transcriptional activator of antibiotic biosynthesis in Streptomyces." Plos Biol., 22, e3002528. doi: 10.1371/journal.pbio.3002528.
- Abstract
- Streptomyces antibiotic regulatory proteins (SARPs) are widely distributed activators of antibiotic biosynthesis. Streptomyces coelicolor AfsR is an SARP regulator with an additional nucleotide-binding oligomerization domain (NOD) and a tetratricopeptide repeat (TPR) domain. Here, we present cryo-electron microscopy (cryo-EM) structures and in vitro assays to demonstrate how the SARP domain activates transcription and how it is modulated by NOD and TPR domains. The structures of transcription initiation complexes (TICs) show that the SARP domain forms a side-by-side dimer to simultaneously engage the afs box overlapping the -35 element and the σHrdB region 4 (R4), resembling a sigma adaptation mechanism. The SARP extensively interacts with the subunits of the RNA polymerase (RNAP) core enzyme including the β-flap tip helix (FTH), the β' zinc-binding domain (ZBD), and the highly flexible C-terminal domain of the α subunit (αCTD). Transcription assays of full-length AfsR and truncated proteins reveal the inhibitory effect of NOD and TPR on SARP transcription activation, which can be eliminated by ATP binding. In vitro phosphorylation hardly affects transcription activation of AfsR, but counteracts the disinhibition of ATP binding. Overall, our results present a detailed molecular view of how AfsR serves to activate transcription.