Summary information and primary citation
- PDB-id
- 8ia3; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (3.5 Å)
- Summary
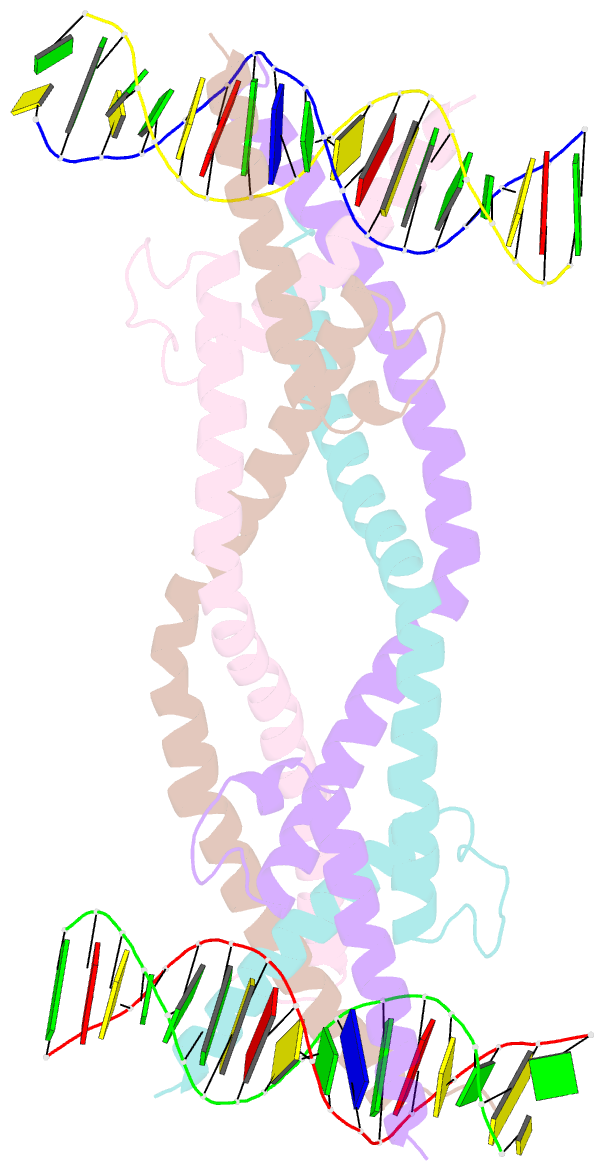
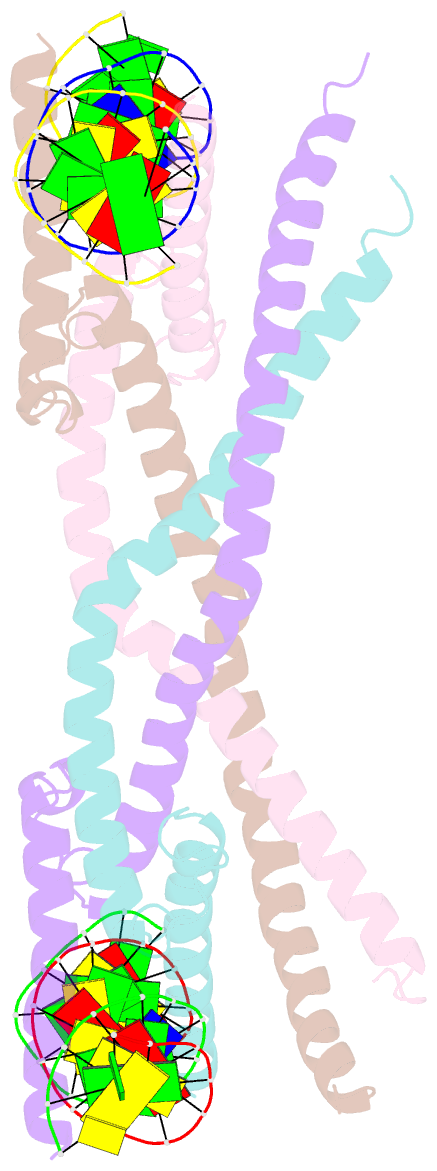
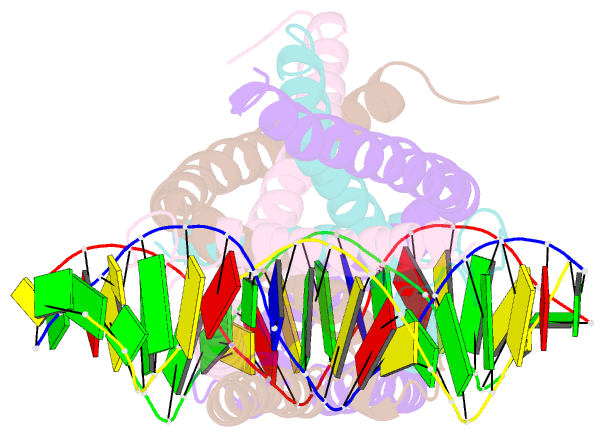
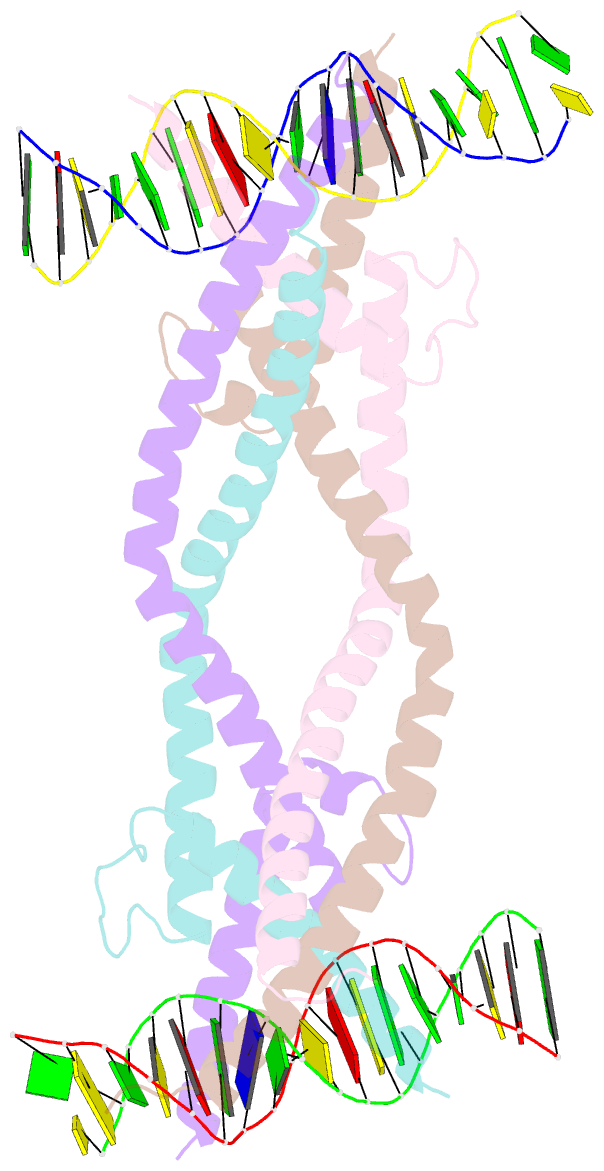
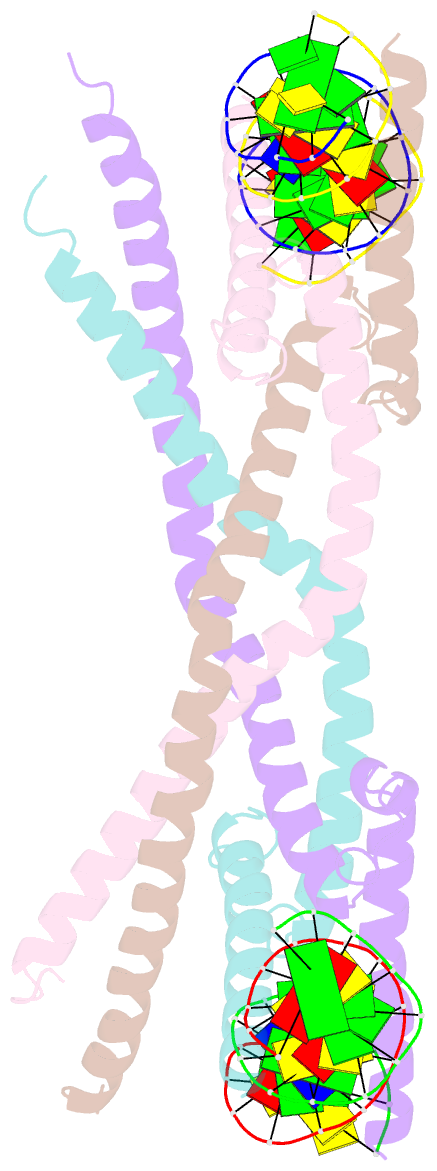
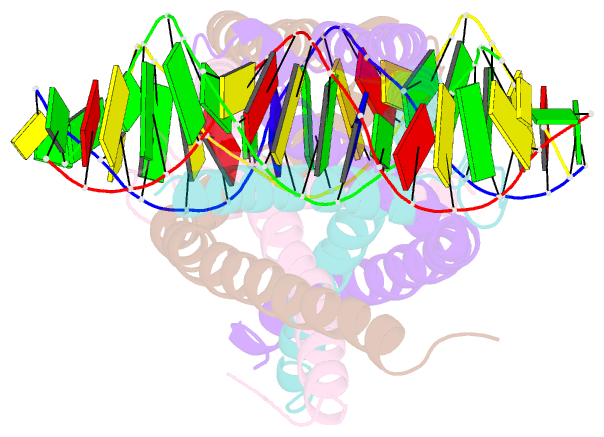
- Crystal structure of human usf2 bhlhlz domain in complex with DNA
- Reference
- Huang C, Xia M, Qiao H, Liu Z, Lin Y, Sun H, Yu B, Fang P, Wang J (2023): "Tetramerization of upstream stimulating factor USF2 requires the elongated bent leucine zipper of the bHLH-LZ domain." J.Biol.Chem., 299, 105240. doi: 10.1016/j.jbc.2023.105240.
- Abstract
- Upstream stimulating factors (USFs), including USF1 and USF2, are key components of the transcription machinery that recruit coactivators and histone-modifying enzymes. Using the classic basic Helix-Loop-Helix Leucine Zipper (bHLH-LZ) domain, USFs bind the E-box DNA and form tetramers that promote DNA looping for transcription initiation. The structural basis by which USFs tetramerize and to bind DNA , however, remains unknown. Here, we report the crystal structure of the complete bHLH-LZ domain of USF2 in complex with E-box DNA. We observed that the LZ of USF2 is longer than that of other bHLH-LZ family transcription factors and that the C-terminus of USF2 forms an additional α-helix following the LZ region (denoted as LZ-Ext). We also found the elongated LZ-Ext facilitates compact tetramer formation. In addition to the classic interactions between the basic region and DNA, we show a highly conserved basic residue in the loop region, Lys271, participates in DNA interaction. Together, these findings suggest that USF2 forms a tetramer structure with a bent elongated LZ-Ext region, providing a molecular basis for its role as a key component of the transcription machinery.